Academic Research Services at ARS Organization
Academic Research Services (ARS) at ARS Organization provides comprehensive research support services, including grant preparation, technical assistance, faculty and staff development, biostatistical support, and more. The organization aims to facilitate research processes and procedures, offering assistance in grant writing, regulatory compliance, and laboratory processing. Contact Yolanda Wess, Eric Smith, Angela Duke, or Emily Dobbs to access these valuable services.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
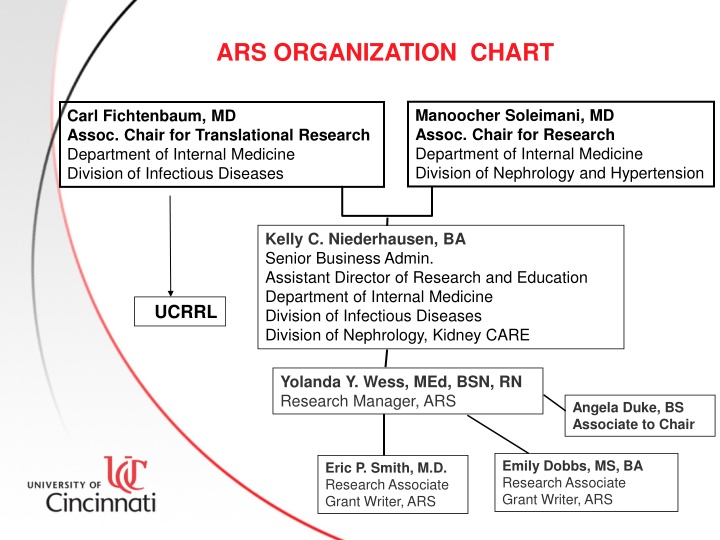
ARS ORGANIZATION CHART Manoocher Soleimani, MD Assoc. Chair for Research Department of Internal Medicine Division of Nephrology and Hypertension Carl Fichtenbaum, MD Assoc. Chair for Translational Research Department of Internal Medicine Division of Infectious Diseases Kelly C. Niederhausen, BA Senior Business Admin. Assistant Director of Research and Education Department of Internal Medicine Division of Infectious Diseases Division of Nephrology, Kidney CARE UUCRRL Yolanda Y. Wess, MEd, BSN, RN Research Manager, ARS Angela Duke, BS Associate to Chair Emily Dobbs, MS, BA Research Associate Grant Writer, ARS Eric P. Smith, M.D. Research Associate Grant Writer, ARS 1
Academic Research Services (ARS) One-stop shop for all things research in DOIM Research Manager Yolanda Wess, MEd Grant support Eric Smith, MD Grant matching- Emily Dobbs, MS Administrative and project support- Angie Duke, BA Services offered Grant preparation, grant matching and support Technical assistance Laboratory processing/shipping Faculty and Staff Development Research processes and procedures Biostatistical support for DOIM researchers Support for trainees Grant education workshops Research conferences Services in the planning stages Regulatory and compliance support
ARS Initial Contact Yolanda Wess at wessyy@uc.edu (513-558-5131), Eric Smith at eric.smith@uc.edu (513-558-2017) Angela Duke at dukeaa@ucmail.uc.edu at (513-558-0827 Emily Dobbs) dobbsek@ucmail.uc.edu at (513-558-7116) Refer to Dr. Jandarov for biostatistical support Refer to Stephanie Williams for Regulatory support Clinical Laboratory processing support Grant writing support Emily Dobbs: Grant Matching Manuscript assistance Review and edit of grant First Introductory Meeting with Eric Smith and Emily Dobbs Will be asked to provide Copy of most current CV/biosketch Kind of grant being submitted- (Funding Opportunity Announcement (FOA) or Request for Application (RFA) or Program Announcement (PA) name and number) Make up of laboratory and/or clinical study personnel support Mentor or collaborator listing Eric Smith Assist with writing, editing and review of grant Grant education and training Careful reading of funding opportunity announcement Generate checklist with timeline, assignment of responsibilities, etc. Plan for unbiased outside reviews though COM pre- review process Arrange for statistical help Assist with manuscript readiness Assist with writing, edit and review of funding applications Refinement of SA, etc. Monitoring timeline and completion of responsibilities Ongoing contact with GA/BA Ensure accurate completion of all science and administrative components of the grant Ensure timely submission of grant to Operations and Finance 5 working days before due date
4 How to access services To initiate support services (Office Location: MSB 6111) Contact: Yolanda Wess at wessyy@uc.edu (513-558-5131) Eric Smith at eric.smith@uc.edu (513-558-2017) Angela Duke at dukeaa@ucmail.uc.edu (513-558-0827) Emily Dobbs at dobbsek@ucmail.uc.edu (513-558-7116) Josie Robinson-Eaton at robinsja@uc.edu (513-558-4287) Either will arrange a meeting, (meeting by phone or in person) to discuss your request A determination will be made if the Academic Research Services (ARS) team can meet the needs within time constraints .
DOIM Biostatistical Resources Expanded Expanded in-house statistician services are now available! The services of Roman Jandarov, PhD are now open to all faculty within our department. Prioritization of requests are for junior faculty and fellows, but all faculty are invited to use these services Services include: Assistance with preliminary studies, power calculations or sample size Advise on research design or data collection methods Data set analysis, general statistical support or creation of data collection instruments Email Dr. Eric Smith, smithep@uc.edu or Yolanda Wess, wessyy@uc.edu (DOIM Academic Research Services (ARS)) to submit your request for DOIM bio stats based services.
UC Retrovirology Reference Lab, (UCRRL) Procedure for accessing service What we do: Who we are: The UC Retrovirology Reference Lab (UCRRL) is a specialty laboratory offering tests concentrated in but not limited to the areas of Molecular Biology, Virology, Immunology, and Pathology. Client services are available to physicians, laboratories, pharmaceutical and clinical trial companies, test manufacturers, and others for the purposes of research, clinical diagnosis, and product/assay comparison/evaluation As a reference laboratory, the UCRRL utilizes approved commercial kits, in- house assays, and products manufactured by other companies following the package inserts and standard laboratory procedures. Assays are well documented in the protocol manual and subject to the appropriate quality control procedures. Tests performed for diagnostic purposes comply with ACTG, UC, ODH, CLIA, and CAP regulations Provide services for laboratory processing of biologic specimens, short-term storage, and shipping for all divisions of the Department of Internal Medicine. The following services are provided by the UCRRL: Process specimens collected by the study coordinator per protocol guidelines Freeze and store specimens per protocol guidelines Batch ship specimens as outlined in the protocol guidelines To obtain a quote for services: Contact the laboratory manager, Josie Robinson-Eaton, at (513) 558-4287. Email the following information: Name and phone number of contact person for on-going communication Name of research coordinator/nurse assigned to the protocol Laboratory manual portion of protocol, Name of the PI for the study Name of the study
SUMMARY 1. ORCID provides a persistent digital identifier that distinguishes you from every other researcher and, through integration in key research workflows such as manuscript and grant submission, supports automated linkages between you and your professional activities ensuring that your work is recognized. 2. Over 7000 journals use ORCID as part of their workflow, and - with the user's permission - can automatically populate ORCID user accounts with citations when they publis 3. NIH applicants can already link SciENcv (Science Expert Network Curriculum Vitae) with their ORCID account to simplify the creation of a biosketch. 4. Those who participate should expect to see additional functionality over time, such as assistance completing NIH applications and reporting requirements as well as allowing public data on NIH grant awards to populate ORCID.
DOIM Research Funding Submission Process Improvements CoM policy on proposal submission guidelines posted. (Policy states that all documents are to be submitted to COM Accounting and Finance five business days prior to the submission deadline, ** upon request permission may be given for final science submission within 2 days of deadline) DOIM has history of late submissions- Currently 42% are late Improvement Plan: CoM Accounting and Finance (A&F) Grant Administrators must be notified of planned upcoming funding submissions as soon as opportunity is identified A &F will notify PI of internal due dates by current CoM SRS policy Beginning December 1, 2017, A& F will pilot 10 day courtesy reminder e- mail for all DOIM. Pilot will be for 3 months. At the internal 5 day deadline a proposal will be considered late Late proposals will be tracked by PI and by document type and division
Ideal timeline Pre-submission planning timeline Months Prior SUBMITTING PLANNING WRITING 8 7 6 5 4 3 2 1 mo. Assess yourself, field, & resources Receipt Date Outline application structure; write your application Set up own review committee; determine human & animal subject requirements Meet institutional deadlines Brainstorm; research idea; call NIH staff Get feedback; edit; proofread
College of Medicine: Grant Pre-Review Program The goal of the CoM Grant Pre-Review Program is to support the refinement of scientific projects and grantsmanship of proposals from CoM faculty prior to official submission of a grant application to a funding agency. The Grant Pre-Review Program accepts electronic submissions of grant proposals on a rolling basis and provides NIH style reviews from our expert faculty. (http://www.med.uc.edu/research/funding/grant-pre-review) Applications for pre-review need to be submitted 60 days prior to the actual grant deadline. The intent of the program is to provide expert reviews within 30 days prior to the submission date of the application
Register for Open Researcher and Contributor ID (ORCID) ORCID, a sort of Social Security number for researchers, is a permanent, unique identifier that can be associated with a researcher's work to resolve any uncertainty about authorship. Nearly 2 million academics have signed up for an ORCID, in total laying claim to over 12 million documents. An ORCID removes author ambiguity especially for individuals with common names or for people who change their name through the course of their career. Certain Publishers and Granting Agencies require that applicants have an ORCID. It helps generate an NIH biosketch more easily A researcher can build an online profile of scholarly works at http://orcid.org. Register directly on the website (http://orcid.org). Upon registering (very easy to do), access your ORCID account using your UC login. Visit the ORCID login page and click the Institutional Account button. Choose University of Cincinnati Main Campus. You will be prompted to link the two accounts.
Other services Other services Intramural funding competitions-(Sr. and Jr. Faculty Pilot, Rehn, Collaborative, Distinguished, Submission Incentive, Research Recognition, Trainee and Post-Doc Travel Awards) Research symposium, poster competition and image gallery Assist with Divisional publications listings and profiles for Annual Report Monthly Research Conferences- September through June Web resources- http://www.med.uc.edu/intmed/research/ars Assist with Biosketches and other grant documents Clinical Trial of the month- IM Internal views