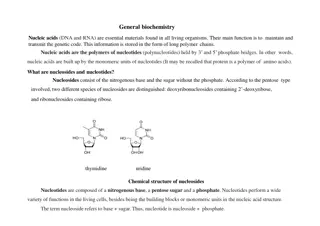
Acids, Bases, and Buffers in Biological Molecules
Explore the concept of acids, bases, and buffers in biological molecules, including acid-base reactions, equilibrium constants, pH definition, conjugate acid-base pairs, pKa values, and titration curves. Gain insights into how functional groups in molecules interact with varying solutions, affecting their properties.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
ACIDS, BASES, AND BUFFERS Dr Manishi Tripathi
ACIDS, BASES, AND BUFFERS Biological molecules, such as proteins and nucleic acids, bear numerous functional groups, such as carboxyl and amino groups, that can undergo acid base reactions. Many properties of these molecules therefore vary with the acidities of the solutions in which they are immersed
AcidBase Reactions 1923 by Johannes Br nsted and Thomas Lowry, an acid is a substance that can donate protons and a base is a substance that can accept protons. acid (here HA) reacts with a base (here H2O) to form the conjugate base of the acid (A) and the conjugate acid of the base (H3O)
The equilibrium constant for the reversible ionization of water is Keq = [H+ ][OH- ] [H2O] In pure water at 25 C, the concentration of water is 55.5 M grams of H2O in 1 L divided by its gram molecular weight: (1,000 g/L)/(18.015 g/mol) and is essentially constant in relation to the very low concentrations of H+ and OH- , namely 1 10-7 M. Accordingly, we can substitute 55.5 M in the equilibrium constant expression to yield
pH pH is defined by
conjugate acid-base pair Equilibrium constants for ionization reactions are usually called ionization constants or acid dissociation constants, often designated Ka
pKa, which is analogous to pH and is defined by the equation A plot of pH against the amount of NaOH added (a titration curve), reveals the pKa of the weak acid. Consider the titration of a 0.1 M solution of acetic acid with 0.1 M NaOH at 25 8C (Fig. 2 17). Two reversible equilibria are involved in the process (here, for simplicity, acetic acid is denoted HAc):
References- Lehninger Principles of Biochemistry, Nelson and Cox, Macmillan Higher education Biochemistry.R.H.Garrett and C.J.Grisham Nelson Education ltd. Biochemistry Voet and Voet