Acute Mesenteric Ischemia in an Elderly Woman
A 71-year-old woman with a history of smoking presented with symptoms of abdominal pain, nausea, vomiting, and diarrhea, raising suspicion of acute mesenteric ischemia. Her medical history, physical examination findings, and diagnostic challenges are explored in this case study.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Presentation A 71-year-old woman with a remote but significant smoking history presented to the emergency department (ED) with 24 hours of abdominal pain, nausea, vomiting, and diarrhea. She was discharged from the hospital 1 week prior after an elective coronary artery bypass graft, which was significant only for an episode of new-onset atrial fibrillation, which resolved after administration of an amiodarone bolus. In the ED, her vitals were as follows: T= 38.1 C, HR 101, BP 148/67, RR 16, and 98% saturation 98% saturation on 2 L of oxygen via nasal cannula.
On physical examination, she was clearly uncomfortable. She had a number of well-healed incisions on her abdomen, including a right subcostal incision from an open cholecystectomy, a left lower quadrant incision from an open appendectomy, a Pfannenstiel incision from two prior cesarean sections, and a lower midline incision from a total abdominal hysterectomy. Her abdomen was obese, distended, and without focal point tenderness. Rectal exam revealed no gross blood, but the stool sample was guaiac positive.
Patient History PMH: Negative PSH: She was discharged from the hospital 1 week prior after an elective coronary artery bypass graft, which was significant only for an episode of new-onset atrial fibrillation , which resolved after administration of an amiodarone bolus. open cholecystectomy, open appendectomy, two prior cesarean sections, abdominal hysterectomy HH: remote but significant smoking history DH: negative CC: Abdominal pain PI: Site: diffuse Onset : 24h ago Character: dull Radiation: none Associating symptoms: nausea, vomiting, diarrhea Time: constant Exacerbation factors: - Severity: 7/10
PH/E: V/S: T= 38.1 C, HR=101, BP=148/67, RR=16, and 98% saturation (on 2 L of oxygen via nasal cannula) General: uncomfortable but not toxic H&N: no pallor, no lymphadenopathy H&L: Normal Abdomen : abdomen was obese, distended, without focal point tenderness, number of well-healed incisions on her abdomen Rectal exam: revealed no gross blood (guaiac positive) Ext: no cyanosis, no bulging
Problem List: Diffuse abdominal pain nausea, vomiting, diarrhea coronary artery bypass graft 1 week ago, for an episode of new-onset atrial fibrillation Distended abdomen without focal point tenderness Tachycardia Low level fever
Orders: Lab: CBC, ABG, Lactate, Troponin & CK-MB, Amylase& Lipase, PT, PTT, INR Imaging: CT angiography
Lab data: WBC: 16,200 cells/mL lactate level : 2.8 mmol/L (elevated) serum bicarbonate level : 19 mEq/L INR :1.1 PTT : 22 seconds PT : 13 seconds A number of laboratory abnormalities are variably present in AMI. Unfortunately, no specific, rapidly available serum marker exists for AMI. Worse yet, most of the markers that do rise in AMI only do so after transmural bowel infarction has already occurred. AMI-associated leukocytosis, lactic acidosis, and serum amylasemia are often seen later in the course of the disease process.
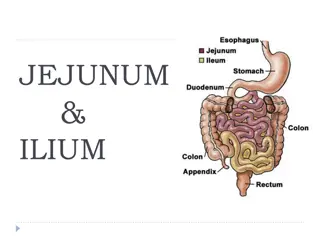
Imaging(CTA): The patient underwent CT angiography (CTA) for further evaluation of her abdominal pain, which revealed an abrupt cutoff in the superior mesenteric artery (SMA) approximately 4 cm distal to the vessel s takeoff from the aorta. The small bowel was dilated, there was evidence of bowel wall thickening, and there was trace free fluid in the pelvis.
CTA has become the most important diagnostic test for acute mesenteric ischemia (AMI). While angiography has been the historic gold standard and has the benefit of allowing for simultaneous endovascular revascularization options, CTA has the advantages of being more readily available and rapid. CTA has demonstrated 93%sensitivity and 96% specificity for the diagnosis of AMI in a large systematic review and meta-analysis. Additionally, CTA also permits simultaneous evaluation of the bowel and the vasculature, as well as allowing for a more thorough evaluation of the abdominal cavity which may rule out other etiologies.
Diagnosis: AMI is a relatively uncommon diagnosis, accounting for <1 in every 10,000 admissions; however, maintaining a high index of suspicion is critical given the high mortality associated with this disease process and a significant delay in diagnosis and treatment can result in a fatal outcome. The mortality rate is 50% when the diagnosis is achieved with 24 hours of clinical presentation, and it climbs to over 70% if the diagnosis is established after that time frame. The missed diagnosis of AMI is a frequent cause of medical malpractice litigation. AMI is typically the clinical manifestation of one of four processes: embolism, thrombosis, nonocclusive mesenteric ischemia (NOMI), or mesenteric venous thrombosis (MVT).
An embolic event, usually to the SMA, is the most common cause of AMI, accounting for approximately half of all cases. Risk factors include atrial fibrillation, congestive heart failure, a history of prior embolic events, and recent myocardial infarction. Acute embolus to the SMA usually lodges in the region of the first branches of the SMA, with the length of small bowel ischemia less affected, usually only portions of the distal jejunum, ileum, and colon.
Arterial thrombosis accounts for 20% of AMI cases. Many of these patients have extensive atherosclerotic disease in the mesenteric vasculature. A thorough history will often reveal abdominal pain after meals (postprandial abdominal pain), weight loss, and food avoidance. Compared with SMA embolism, which tend to occur slightly more distal, SMA thrombosis typically occurs at the origin of the vessel, which creates ischemia from the mid-duodenum to the splenic flexure Acute thrombotic occlusion of the SMA usually occurs secondary to a proximal atherosclerotic plaque, yielding extensive ischemia of the entire small bowel and large bowel.
Nonocclusive mesenteric ischemia (NOMI) NOMI results from a relative low-flow state and vasoconstriction, creating an imbalance between mesenteric oxygen supply and demand. NOMI accounts for 20% of all presentations of AMI. These patients typically have a greater burden of atherosclerotic disease and are critically ill. These contributing factors predispose the patient to the development of impaired intestinal perfusion in the absence of occlusion of the celiac, superior mesenteric, and inferior mesenteric arteries. CTA usually demonstrates narrowing of multiple branches of the SMA, alternating dilation and narrowing of the mesenteric vessel, spasm of the SMA mesenteric arcades, and impaired filling of intramural vessels. CTA often will show a flattened inferior vena cava, consistent with the low-flow etiology of NOMI.
Mesenteric venous thrombosis (MVT) MVT is the least common cause of AMI and accounts for approximately 10% of all causes. This ischemia is secondary to impaired venous outflow resulting in venous congestion and engorgement. MVT is typically caused by primary or idiopathic thrombosis with 90% of cases related to thrombophilia, traumatic injury, or local inflammatory process (i.e., acute pancreatitis, acute diverticulitis, inflammatory bowel disease, or infections within the biliary system). Significant ascites is often appreciated on imaging.
Diagnosis For the patient in our particular scenario, the recent history of atrial fibrillation, cardiac disease, leukocytosis, low-grade fever, tachycardia, lactic acidemia, and pain out of proportion to physical exam, an emergent exploratory laparotomy was indicated.
Treatment In general, the cornerstones of treatment for AMI include fluid resuscitation, correction of electrolyte abnormalities, intravenous antibiotics, initiation of therapeutic anticoagulation, immediate revascularization, and resection of irreversibly necrotic bowel.
Post operative Patients should be continued on systemic heparin therapy in the immediate postoperative period. All patients will require anticoagulation and/or antiplatelet therapy. Because these patients tend to have systemic vascular disease, they require aggressive risk factor modification as much as possible.
Case Conclusion The patient underwent an exploratory laparotomy, which revealed extensively threatened bowel from the jejunum through the ascending colon. An embolectomy was performed, which reestablished blood flow after the removal of a large embolus. The patient s abdomen was left open with a wound vacuum system providing a temporary abdominal closure. She returned to the Surgical Intensive Care Unit for aggressive resuscitation. Approximately 36 hours later, she returned to the operating room for a planned second-look procedure. Much of the previously threatened bowel demonstrated significant improvement, though she still required resection of the distal ileum. At that time, her abdomen was definitively closed. She was discharged to a rehabilitation facility on postoperative day seven, with warfarin anticoagulation, aspirin, a statin, and monthly vitamin B12 injections to compensate for the distal ileum resection.