Addressing Antimicrobial Resistance Challenges and Innovations in the UK
Explore the urgent need for new antimicrobials to combat rising drug resistance, the UK's vision for tackling antimicrobial resistance by 2040, and the current market failures hindering progress. Learn about the innovative models being developed to evaluate and purchase antimicrobials, and the importance of investing in novel solutions to address this pressing global challenge.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
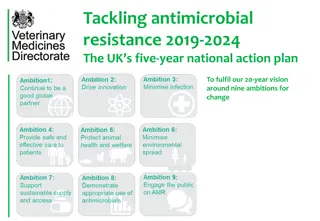
Antimicrobial Resistance: Developing and testing innovative models for the evaluation and purchase of antimicrobials. In support of the UK s vision for AMR by 2040 and five-year national action plan Spring 2020
Clinical context and need for action Antimicrobial resistance (AMR) is one of the most pressing global challenges we face this century. Although the UK has made progress in reducing the use of antimicrobials in humans and animals in the past five years, drug resistant bloodstream infections in humans increased by 35% from 2013-2017 in England. https://www.gov.uk/government/news/antimicrobial- resistance-uk-launches-5-year-action-plan-and-20-year-vision For most antimicrobials, there are few replacements or alternative products in development and even fewer that target priority pathogens. Investment in novel antimicrobials is widely seen as commercially unattractive the high R&D costs and low returns have led to market failure. The increased use of antimicrobials to cover potential secondary bacterial infection in COVID-19 pneumonia and the emergence of bacterial resistance from this increased usage further emphasise the need for a reinvigorated antimicrobial pipeline. 2
UKs vision for AMR by 2040 and 5-year national action plan Published on 24 January 2019. Our vision is of a world in which AMR is effectively contained, controlled and mitigated by 2040. The national action plan commits to lead the way in testing solutions that will address our global failure to incentivise the development of new antimicrobials and alternative treatments. https://www.gov.uk/government/collections/antimicrobial-resistance-amr-information-and-resources#strategic-publications 3
Market Failure The market for a new antimicrobial following launch may be limited in two main ways: New products active against priority pathogens should be subject to strict stewardship and used on a last in line basis. In the absence of significant outbreaks of drug-resistant infections, sales could be minimal. New products offering alternative options to existing antibiotics, which could potentially be valuable as part of cycling stewardship regimes, tend not to be used due to their high cost compared to existing products (many of which are generics). 4
Current pipeline Only 42 new antimicrobial compounds in phase I-III clinical trials (March 2019) https://www.pewtrusts.org/en/research-and-analysis/issue-briefs/2019/09/tracking-the-global-pipeline-of-antibiotics-in- development Compared with 1,571 immuno-oncology agents in development (September 2019) https://www.cancerresearch.org/scientists/immuno-oncology-landscape There is an urgent need to increase investment 5
The project Sponsored by: NICE and NHS England & NHS Improvement Aim: to demonstrate the feasibility of innovative models that pay companies for antimicrobials based primarily on a health technology assessment of their value to the NHS as opposed to the volumes used. 6
Background Joint Government/ Industry AMR working group established 2015. A sub-group focussing on reimbursement and evaluation. Principle of an innovative payment model broadly agreed. Commissioned the Economic Evaluation Policy Research Unit (EEPRU), to consider evaluation in 2017. EPPRU reported October 2018 scope for a pragmatic HTA framework informed by health economic modelling and expert opinion. UK five-year national action plan for AMR January 2019 commits to test a new model. 7
The model The model would forecast the value of health benefits provided by a new antimicrobial Value will be estimated by NICE through an adapted Health Technology Assessment with information from health economic modelling and expert opinion. The model will capture value not just from the direct health gain to patients treated, but additional elements: e.g. insurance value, diversity value, transmission value and enablement value Value forecast used to inform commercial negotiations, leading to payments to the company in instalments. A one size fits all model is unlikely to be possible may need to be tailored to suit particular medicines, pathogens and settings including unmet need and future threats. 8
Application of the model The model will be tested through application to two antimicrobial products: One existing (has received marketing authorisation in past 2-3 years) One new to market (with expectation of a marketing authorisation by end 2020 and with plans to launch in UK) Future policy for the evaluation and purchasing of antimicrobials will be informed by the outcomes from this project. 9
Project outline 1 - Develop outline methods for product selection, evaluation and commercial model. 6. Implement payments 5. Commercial negotiation of payments for the selected products 3. Selection of 2 products to test via competitive dialogue 4. Value assessment for the selected products 2 - Develop detailed documentation for procurement process 7. Monitor 8. Evaluate 9. Communicate 10
Progress July 2019: Project launched. September 2019: Completion of stakeholder engagement on draft evaluation framework, product selection process and commercial model. October 2019: Stakeholder webinar covering outcomes from the targeted engagement and outline procurement process. February 2020: Project Advisory Group recruited. March 2020: Draft documents for procurement process (via competitive dialogue route) shared with stakeholders. March 2020: Webinars held with companies and broader stakeholders to launch and support market engagement. June 2020: Feedback from the market engagement provided to stakeholders and procurement documents finalised. 11
Next steps Launch the procurement via Official Journal of the European Union notification (Spring/Summer 2020). Select two products for the test through competitive dialogue (by end Dec 2020). Undertake value assessment for selected products (through 2021). Complete commercial negotiation to agree the level of subscription payments (early 2022). Implement subscription payments (Spring 2022). Monitor progress and disseminate learning throughout. 12
Success An agreed HTA valuation framework and complete value assessment of two products. An agreed payment framework that leads to successful negotiation of payments for the two products, supporting good stewardship. Other countries test models that, together, achieve pull incentives for antimicrobials and stimulate companies to increase investment. 13