Advanced Reaction Kinetics and Mechanisms Overview
Learn about nonelementary reaction kinetics, postulating reaction mechanisms, deriving rate equations, and understanding free radical polymerizations. Discover how to determine complex reaction mechanisms based on experimental observations.Slides provided by Prof. M.L. Kraft, University of Illinois, Urbana-Champaign.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
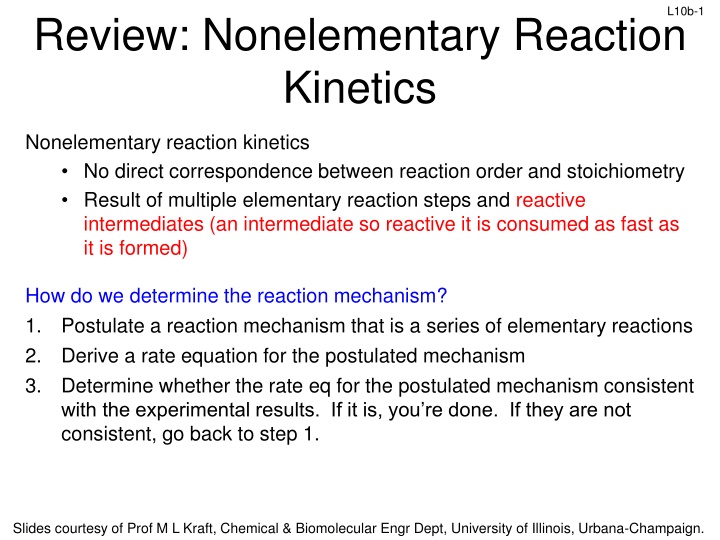
L10b-1 Review: Nonelementary Reaction Kinetics Nonelementary reaction kinetics No direct correspondence between reaction order and stoichiometry Result of multiple elementary reaction steps and reactive intermediates (an intermediate so reactive it is consumed as fast as it is formed) How do we determine the reaction mechanism? 1. Postulate a reaction mechanism that is a series of elementary reactions 2. Derive a rate equation for the postulated mechanism 3. Determine whether the rate eq for the postulated mechanism consistent with the experimental results. If it is, you re done. If they are not consistent, go back to step 1. Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign.
L10b-2 Review: Postulating a Reaction Mechanism Based on an Experimentally Observed Rate Law If CB appears in the denominator of the experimentally observed rate law, then one elementary reaction step is probably: * B A Collision products + 1. where A* is a reactive intermediate 2. If the denominator contains a constant (by itself, not multiplied by a concentration), then one reaction step is probably: * A Decomposition products 3. If the numerator contains a species concentration, then one rxn step is probably: species C other species? + * + A other products? Derive a rate equation for the postulated mechanism and check if it describes the experimentally observed rate equation This will definitely be on quiz 3! Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign.
L10b-3 Review: Deriving a Rate Equation for a Postulated Mechanism 1) Write rate equation for postulated mechanism ( ) ( ) = A r Ar rxns that form A - rxns that consume A ( ) rxns that form A - rxns that consume A = ( ) 2) For concentrations of reactive intermediates CI* that appear in the rate equation rA a) Write out the rate equation for reactive intermediates rI* b) Apply Pseudo-Steady State Hypothesis, which states that the net formation of reactive intermediates is zero (rI*=0) c) Solve for CI* in terms of measurable species d) Substitute the new expression for CI* in terms of measurable species back into -rA 3) Rearrange rA to check if it matches the experimentally observed rate equation Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign.
L10b-4 Review: Free Radical Polymerizations M monomer M M M k0 polymer M-M-M M M n 1. Initiation: Initiator (I) decomposes to 2 free radicals Radical (1) 2I + 2I ki I M R 1 kp 2. Propagation: + kp R M R Chain elongation, new monomers add to chain 1 2 + R M R+ j j 1 km 3. Chain transfer: + + R M P R j j 1 Live polymer chain transfers radical to monomer. Polymer chain is no longer reactive (dead). Can also transfer to solvent or other species kadd j k R R + 4a. Termination by addition: R+ j k kdis + + 4b. Termination by disproportionation: R R R R j k j k Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign.
L10b-5 Review: PSSH Applied to Thermal Cracking of Ethane The thermal decomposition of ethane to ethylene, methane, butane and hydrogen is believed to proceed in the following sequence: = k1 1,C H r 1 C H k C Initiation: 2 6 C H 2CH 2 6 2 6 3 k2 = 2,C H r 2 CH k C C Propagation: + + CH 2 6 C H CH 2 5 C H C H 2 6 2 6 3 3 4 k3 = 3,C H r 3 C H k C + 2 5 C H 2 4 C H H 2 4 2 5 k4 = 4,C H r 4 H k C C ( 5 H C H + + 2 5 C H H C H 2 6 2 6 2 6 2 ) 2 k5 2C H 4 10 C H Termination: = 5,C H r k C 2 5 C H 2 5 2 5 (a) Use the PSSH to derive a rate law for the rate of formation of ethylene (b) Compare the PSSH solution in Part (a) to that obtained by solving the complete set of ODE mole balance Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign.
L10b-6 L10b: Bioreactors Today s goals: Predict rates of enzyme-catalyzed reactions Determine effect of chemical inhibitors on rxn rate Develop mathematical expression based on fundamental steps of rxn Apply model to cell growth Enzymes: Protein catalyst that execute complex biochemical reactions- all synthetic and degradative reactions in living organisms! Increases the rate of reaction without undergoing permanent chemical change not used up (consumed) by the reaction Substrate: the reactant that the enzyme acts on Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign.
L10b-7 Enzymes Increase Reaction Rate Effects the reaction rate (kinetics), NOT equilibrium (thermo) Lower activation energy G increases reaction rate, reach equilibrium faster G is unchanged, so ratio of products to reactants at equilibrium is the same k1 + Kinetic : A B C Activation energy for uncatalyzed reaction k1 k1,cat>k1,uncat Activation energy for catalyzed rxn G determines rxn rate G = -RT ln(k) Enzymes change G Transition state, G Thermodynamic: k k [C] Enzymes do NOT change G 1 = = K A B = -1 K K cat uncat Gdetermines equilibrium G= -RT ln(K) Enzymes do NOT change G Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign.
L10b-8 Michaelis-Menten (M-M) Equation Vmax: maximum reaction rate, further increases in substrate, S, no longer increase the reaction velocity, v [rP] v = reaction velocity = rP = -rS Km = substrate concentration where reaction velocity v = Vmax/2 [S] = substrate concentration [P]: product concentration C C V K Empirically found the Michaelis-Menten equation: max S + = v m S Where Vmax depends on the amount of enzyme Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign.
L10b-9 Rate Equation for Enzymatic Reaction S V v r S K + max = = Goal: derive this experimentally determined reaction rate P m k E: enzyme ES: enzyme-substrate complex S: substrate k 1 2 + + E S ES E P k1 dC dt P = = = rate of product formation: v P r C k 2 ES We cannot measure CES, so we need to get CES in terms of species we can measure. Start by writing the rate equation for CES : dC ( ) ES dt = + S E C C C k k k 1 1 2 ES The free enzyme concentration CE is also difficult to measure. Use the mass balance to get CE in terms of CES and CE0. C C C where C = Substitute into rate eq for CE: = C = E E0 ES E0 E,t 0 dC ( ) ( ) ES dt = + C C C C k k k 1 1 2 S E0 ES ES Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign.
L10b-10 CES in Measurable Quantities ( 1 S E0 C C k dt dC ) ( ) ES = + C C k k 1 2 ES ES d ES dt Pseudo-steady state assumption: ES is a reactive intermediate, so = 0 dC ( ) ( ) ES dt Now solve for CES = = + 0 C C C C k k k 1 1 2 S E0 ES ES + = C C S E0 C C S ES C C k k k k Multiply out and rearrange 1 2 1 1 ES ES + + = C C S ES C C S E0 C C k k k k Bring CES to left side of equation 1 2 1 1 ES ES ( ) + + = C C S E0 C C k k k k Factor out CES 1 2 1 1 ES S 1 S E0 C C k + Plug this expression for CES into dCP/dt k = C Divide by quantity in bracket ES + 1 S C k k 1 2 C C S E0 2 k + = Divide top & bottom by k1 C ES k 1 k + C S 1 Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign.
L10b-11 Derivation of the M-M Equation k k 1 2 k1 E: enzyme ES: enzyme-substrate complex S: substrate + + E S ES E P dC dt P = = = rate of product formation: v P r C k 2 ES C C Plug this expression for CES into dCP/dt S E0 2 k + = C ES k 1 k + C S 1 C + C dC dt k Compare to experimentally observed rate eq: 2 E0 1 k k C = C C S P V K = = P r ma x S = = v P r k k 2 + + C m S S 1 When CS>>Km, then: max V 2 E0 = P r r max V Vmax occurs when enzyme is fully saturated with S (in ES form) s When CS<<Km, then: C + V k k max S K 1 k 2 = = P r S r = K m m 1 Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign.
Complications with Measuring Rates with the M-M Equation L10b-12 In practice, Vmax can be difficult to estimate using the MM equation. Everyone reported different values of Vmax. Since a solution with infinite concentration of substrate is impossible to make, a different equation was needed. Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign.
L10b-13 Lineweaver-Burk Equation Lineweaver & Burk inverted the MM equation P K C 1 r C V C C V K max S + = r m S + m S = max P S K 1 r 1 1 m = + C V V max max p S ( ) ( ) m x = + y b By plotting 1/ v vs 1/CS, a linear plot is obtained: Slope = Km/Vmax y-intercept = 1/Vmax x-intercept= -1/Km 13 Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign.
L10b-14 Types of Reversible Inhibition Substrate 1. Competitive: I is the inhibitor Competitive inhibitor Binds to active site & blocks substrate binding Reduces the CEnzyme available for binding 2. Noncompetitive Substrate K m KI KI Inhibitor binds to some other site Does not affect substrate binding K m Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign.
L10b-15 1. Competitive Inhibition Competitive inhibition No inhibition Substrate and inhibitor compete for same site max V + C C C V max S S = = r vs r p p + K C K m S I + K 1 C m S Km, app >Km Vmax, app =Vmax I I K Kmobserved w/ competitive inhibitor = + K K 1 m,app m K 1 1 1 I m = + P r C V V 14 max max S Will reach Vmax of uninhibited rxn at high CS velocity (mmol/min) 1 12 Uninhibited reaction 10 0.8 Inhibited reaction 8 0.6 6 1/rP 0.4 4 Inhibited reaction Uninhibited reaction 2 0.2 0 0 0 10 20 CS (mM) 30 40 50 -1 0 1/CS (mmol)-1 1 2 -0.2 Can be overcome by high substrate concentration Slope = Km/Vmax y-int = 1/Vmax x-int= -1/Km Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign.
L10b-16 2. Noncompetitive Inhibition Competitive inhibition No inhibition ( ) ( ) + max V 1 C K + C C C V K I I S ma x S = = v = r vs r P p + C K S V m m S Vmaxobserved w/ noncompetitive max V + substrate and inhibitor bind different sites = max,app C K inhibitor I 1 Increasing CI I No I 1 r Vmax p Vmax,app CI rP K m = m V Vmax,app max,app higher CI 1 = y int max,app V 1 C 1 CS Km K S m Vmax, app < Vmax Km, app = Km K 1 1 1 m = + P r m,app V C m,app V S Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign.
L10b-17 3. Uncompetitive Inhibition E S I ( ) ( K ) + V 1 C K C C C V ma + x I I S ma x S = v = r = vs r p p ( ) + + C 1 C K K substrate & inhibitor bind different sites but I only binds after S is bound S m I I m S max V max,app V = C K I + 1 I K m C K K = m,app I + 1 I 1 = y int max, app V Vmax, app < Vmax Km, app <Km 1/rp K V m,app = slope K 1 1 1 m,app max,app = + P r max,app V C max,app V 1 S 1/CS = x-int K m, app Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign.
L10b-18 Batch Bioreactor or Fermentor S0 C represents cell mass (biomass) well stirred reaction limited C S C S C S S S S C C S Batch Reactor In a reactor with substrate at concentration CS0 and CC =0 add cells at concentration CC0 at t = 0 add no more S or cells after t = 0 monitor CS and CC with time time t=0 s Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign.
L10b-19 Kinetics of Microbial Growth (Batch or Semi-Batch) Region 1:Lag phase microbes are adjusting to the new substrate Region 2: Exponential growth phase microbes have acclimated to the conditions Region 3: Stationary phase limiting substrate or oxygen limits the growth rate Region 4: Death phase substrate supply is exhausted CC,max log [X] Log CC 1 2 3 4 CC0 Time Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign.
L10b-20 Quantifying Growth Kinetics Relationship of the specific growth rate to substrate concentration exhibits the form of saturation kinetics Assume a single chemical species, S, is growth-rate limiting Apply Michaelis-Menten kinetics to cellular system called the Monod equation C C max K S = Monod equation: r C g C + s S max is the maximum specific growth rate when S>>Ks CS is the substrate concentration CC is the cell concentration Ks is the saturation constant or half-velocity constant. Equals the rate- limiting substrate concentration, S, when the specific growth rate is the maximum Semi-empirical, experimental data fits to equation, assumes that a single enzymatic reaction, and therefore substrate conversion by that enzyme, limits the growth-rate Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign.
L10b-21 Monod Model (1942) Nobel Prize First-order kinetics: m S C + Zero-order kinetics: m S C K = r C = C K r C g C = S S g C C K r K C s S S S g m s m Exponential phase decelerating phase KS CS Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign.
Mass Balance on Cell Growth with Products Overall balance for cells growing on carbohydrate with products: CHmOn + a O2 + b NH3 c CH N + d CHxOyNz + e H2O + f CO2 L10b-22 Product Carbohydrate (can be any organic material) Nitrogen source Cell material (biomass) Individual elemental balances: 1) Carbon: 2) Hydrogen: m + 3b = c + dx + 2e 3) Oxygen: n + 2a = c + dy + e + 2f 4) Nitrogen: b = c + dz 1 = c + d + f Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign.
L10b-23 Yield Coefficients C S cell mass formed = Cell yield for substrate: C/S Y substrate consumed C O cell mass formed Cell yield for O2: = Y C/O2 oxygen consumed 2 P product mass formed Product yield for substrate: = Y P / S S substrate consumed Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign.