Advanced Techniques in Antibiotic Microencapsulation
Explore innovative methods such as spray-drying and emulsification for microencapsulating antibiotics like erythromycin, clarithromycin, and rifampicin. Learn how these techniques enhance drug delivery, reduce toxicity, and maintain optimal drug levels. Discover the development of encapsulated antibiotics for wound care, topical applications, and other therapeutic agents.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
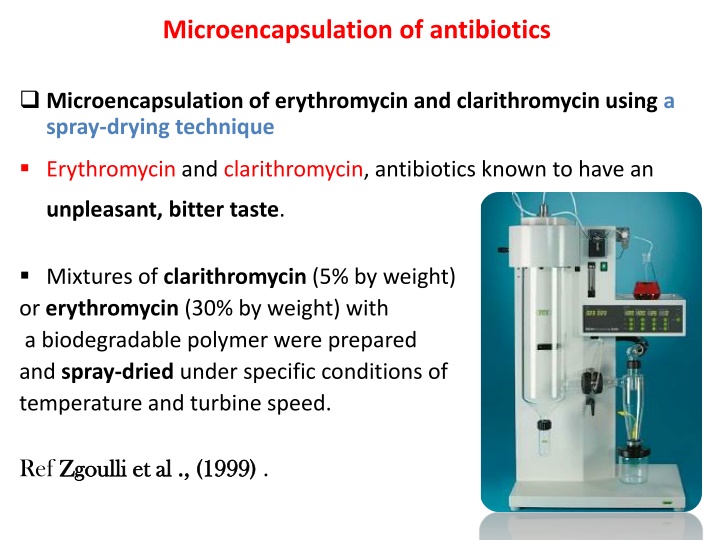
Microencapsulation of antibiotics Microencapsulation of erythromycin and clarithromycin using a spray-drying technique Erythromycin and clarithromycin, antibiotics known to have an unpleasant, bitter taste. Mixtures of clarithromycin (5% by weight) or erythromycin (30% by weight) with a biodegradable polymer were prepared and spray-dried under specific conditions of temperature and turbine speed. Ref Zgoulli Zgoulli et al ., (1999) et al ., (1999) .
This process resulted in the microencapsulation of 80% of each drug .Particle size ranged from 1 to 80 mum . Microencapsulation of antibiotic rifampicin in poly(3- hydroxybutyrate-co-3-hydroxyvalerate) The poly(3-hydroxybutyrate-co-3-hydroxyvalerate) microparticles were prepared by the emulsification and solvent evaporation method. It is used to increase the drug concentration at the site of infection , decrease the toxicity of the drug and maintenance of drug levels within a desired range . Ref Dur n Dur n et al., ( et al., (2008 2008) . ) .
Other forms of rifampicine encapsulation ; o PLGA microspheres containing rifampicine or isoniazid have been successfully prepared for the direct delivery to the lung . It could improve the antibacterial efficacy but unfortunately, the cost of this polymer is too high . o Studies of tuberculosis using nanocapsules of poly(n- butylcyanoacrylate) and poly(butylcyano acrylate) with isoniazid, rifampicine and streptomycin has improved drug bioavailability and reducing the dosing frequency for better management of pulmonary tuberculosis. Ref Dur n Dur n et al., ( et al., (2008 2008) . ) .
Development of Encapsulated Antibiotics for Topical Administration to Wounds ; Ref Setterstrom et al., (1984) To prevent infection, systemic antibiotics must be administered within four hours after wounding when circulation is optimal . If treatment is delayed, a milieu for bacterial growth develops . The DL-PLG copolymer is well suited for in vivo drug release because it elicits a minimal inflammatory response. Other types of antibiotics microencapsulation ; Lupin has already launched in the market worlds first Cephalexin (Ceff-ER) and Cefadroxil (Odoxil OD) antibiotic tablets for treatment of bacterial infections. Ref Dubey Dubey, R. ( , R. (2009 2009). ).
Microencapsulation of therapeutic agents ; Microencapsulation of Biological Agents, Food Supplements& Enzymes; Biodegradable and biocompatible polymers, such as PLGA, have been used to encapsulate agents such as risperidone (antipsychotic) and testosterone to form microparticles. Also vaccine formulations are developed , anti-oxidants as vitamin C and gallic acid , -agonists, anticholinergics, mucolytics, and antimicrobials,
Microencapsulation of Anticancer Drugs and Genes As cisplatin Ethyl cellulose by phase separation used to encapsulate highly hydrophilic drugs such as (NSAIDs) and diclofenac sodium, used for the treatment of rheumatoid arthritis ,anti-diabetic as glipized . Microencapsulation of Proteins and Hormones high-molecular- weight proteins are poorly absorbed by the blood stream and are also sensitive to degradation by the acidic environment of the GIT. Examples Such as LH , (LHRH) , growth hormone ,calcitonin and insulin all are encapsulated .
Other non-pharmaceutical applications of microencapsulation Agricultural : Reduce insect population by disrupting their mating process Protects the pheromone from oxidation and light during storage and release Catalysis : Safe handling ;easy recovery ;reuse and disposal at an acceptable economic coast
Food industry Sometimes they slowly degrade and lose their activity or become hazardous by oxidation reactions ingredients can also react with components present in the food system The food industry utilizes functional ingredients to improve flavor, color, and texture properties and to extend the shelf- life of products
Packaging Packaging is defined as the collection of different components which surround the pharmaceutical product from the time of production until its use. Packaging is responsible for providing life-saving drugs, medical devices, medical treatments, and new products like medical nutritionals (nutraceuticals) . Ref(9), Michael E. Aultonand Kevin M. G. Taylor(2013) , Aulton Pharmaceutics The Design and Manufacture of Medicine Pharmaceutics The Design and Manufacture of Medicine Aulton s s
containment Convenience protecion Function of packaging Presentation Identification & information Ref(9), Michael E. Aultonand Kevin M. G. Taylor(2013) , Aulton Pharmaceutics The Design and Manufacture of Medicine Pharmaceutics The Design and Manufacture of Medicine Aulton s s
Types of packaging : Primary packaging system is the material that first envelops the product and holds it i.e., those package components and subcomponents that actually come in contact with the product, or those that may have a direct effect on the product shelf life e.g., ampoules and vial, and IV containers. Secondary packaging system is outside the primary packaging and used to group primary packages together e.g., cartons, boxes and shipping containers . Tertiary packaging system is used for bulk handling and shipping e.g., barrel, container, edge protectors, etc. Ref(8) , Nityanand Zadbuke, Sadhana Shahi, Bhushan Gulecha, Abhay Padalkar, and Mahesh Thube (2013) , Recent trends and future of pharmaceutical Recent trends and future of pharmaceutical packaging technology packaging technology,
Ref(9), Michael E. Aultonand Kevin M. G. Taylor(2013) , Aulton Pharmaceutics The Design and Manufacture of Medicine Pharmaceutics The Design and Manufacture of Medicine Aulton s s
Materials used for Packaging of microencapsulation : The majority of medicines (51%) have been taken orally by tablets or capsules, which are either packed in blister packs or fed into plastic bottles . Closures : ref (2) , Aulton s Pharmaceutics 1. A closure is a device e.g. stopper, lid, top or cap which is used to close a container and is an integral part o the pack. Materials must be sterilized and must not interact with the inner ingredients of the micro encapsulated drug . Humidity and temperature inside must be adjusted . Ref(1) Packaging Technologies as shown in the following video, https://www.youtube.com/watch?v=4ShVXPbI9Mw .
Storage A capsule is a very sensitive product and it must be protected against excessive heat and humidity For storage we recommend a temperature between 15 to 25C and a relative humidity between 35 and 65% Do not leave open inner bags in non air conditioned areas. Cartons must be sealed and bags should never be left open in areas where the air is not controlled
References References 1 ) Zgoulli, S., Grek, V., Barre, G., Goffinet, G., Thonart, P. H., & Zinner, S. (1999). Microencapsulation of erythromycin and clarithromycin using a spray-drying technique. Journal of microencapsulation Journal of microencapsulation, 16(5), 565-571. https://doi.org/10.1080/026520499288762 Dur n, N., Alvarenga, M. A., Da Silva, E. C., Melo, P. S., & Marcato, P. D. (2008). Microencapsulation of antibiotic rifampicin in poly (3-hydroxybutyrate-co-3- hydroxyvalerate). Archives of Archives of pharmacal pharmacal research https://link.springer.com/article/10.1007/s12272-001-2137-7 research, 31(11), 1509-1516. 3 ) Setterstrom, J. A., Tice, T. R., & Myers, W. E. (1984). Development of encapsulated antibiotics for topical administration to wounds. In Recent advances in drug delivery systems systems (pp. 185-198). Springer, Boston, MA. https://link.springer.com/chapter/10.1007/978-1-4613-2745-5_12 Recent advances in drug delivery 4 ) Dubey, R. (2009). Microencapsulation technology and applications. Defence Journal Journal, , 59(1), 82. http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.849.9194&rep=rep1&type=pdf Defence Science Science
5) Catherine Tomaro-Duchesneau,1Shyamali Saha,1,2Meenakshi Malhotra,1Imen Kahouli,1,3and Satya Prakash(2013) ,Microencapsulation for the Therapeutic Delivery of Drugs, Microencapsulation for the Therapeutic Delivery of Drugs, Live Mammalian and Bacterial Cells, and Other Biopharmaceutics: Current Status and Future Live Mammalian and Bacterial Cells, and Other Biopharmaceutics: Current Status and Future Directions Directions , http://dx.doi.org/10.1155/2013/103527. 6) http://www.agrsci.jp/ras/article/view/23/47 7)R. Dubey , Microencapsulation Technology and Applications , Defence Scince Journal, Vol. 1 . January 2009, Page no. -82-95. 8) Nityanand Zadbuke, Sadhana Shahi, Bhushan Gulecha, Abhay Padalkar, and Mahesh Thube (2013) , Recent trends and future of pharmaceutical packaging technology Recent trends and future of pharmaceutical packaging technology, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3697200/ . 9) Michael E. Aultonand Kevin M. G. Taylor(2013) , Aulton Pharmaceutics The Design and Manufacture of Medicine Pharmaceutics The Design and Manufacture of Medicine , file:///C:/Users/Do7a/Downloads/docslide.net_aultons-pharmaceutics-the-design-and- manufacture-of-medicines-4th-ed%20(1).pdf. Aulton s s 10) Leon, L ., Herbert A. L., Joseph, L. K; The Theory And Practice Of Industrial Pharmacy , 3rd edition (1990). Varghese Publishing House , Page no, -412-428.