AFLIBERCEPT
Aflibercept, with a molecular weight of 115 kDa, is used for wet AMD, macular edema following CRVO, and metastatic colorectal cancer. It acts as a decoy receptor for VEGF-A and PIGF, inhibiting neovascularization and vascular permeability. The drug has high affinity for VEGF-A and is administered intravitreally. Its systemic form, ziv-aflibercept, in combination with FOLFIRI, is indicated for resistant metastatic colorectal cancer. Aflibercept's pharmacodynamics and metabolism suggest therapeutic efficacy and safety in its indicated conditions.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
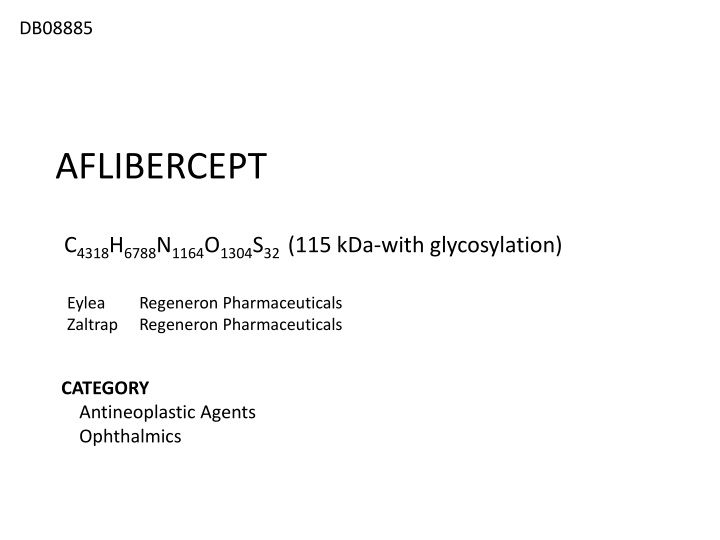
DB08885 AFLIBERCEPT C4318H6788N1164O1304S32 (115 kDa-with glycosylation) Eylea Zaltrap Regeneron Pharmaceuticals Regeneron Pharmaceuticals CATEGORY Antineoplastic Agents Ophthalmics
DESCRIPTION Aflibercept is a recombinant fusion protein that comprises of two main components: the vascular endothelial growth factor (VEGF) binding portions from the extracellular domains of human VEGF receptors 1 and 2 which is then fused to the Fc portion of human IgG1. Structurally, Aflibercept is a dimeric glycoprotein with a protein molecular weight of 96.9 kilo Daltons (kDa). It contains approximately 15% glycosylation to give a total molecular weight of 115 kDa. All five putative N-glycosylation sites on each polypeptide chain predicted by the primary sequence can be occupied with carbohydrate and exhibit some degree of chain heterogeneity, including heterogeneity in terminal sialic acid residues, except at the single unsialylated site associated with the Fc domain. Aflibercept, as an ophthalmic agent, is used in the treatment of macular edema following Central Retinal Vein Occlusion (CRVO) and neovascular Age-Related Macular Degeneration (AMD). Ziv- aflibercept, under the brand name Zaltrap, was developed as an injection for treatment of metastatic colorectal cancer. FDA approved in November 18, 2011 and EMA approved in November 2012.
INDICATION The opthalmic agent is used for the treatment of neovascular (wet) age-related mascular degeneration (AMD) and macular edema following central retinal vein occulsion (CRVO). The systemic injection, known as ziv-aflibercept, in combination with 5-fluorouracil, leucovorin, irinotecan-(FOLFIRI), is for the treatment of metastatic colorectal cancer that is resistant to or progressed following treatment with oxaliplatin. PHARMACODYNAMICS Compared to other anti-VEGF drugs like bevacizumab and ranibizumab, aflibercept has a higher binding affinity to VEGF-A (Kd = 0.5 pM). MECHANISM OF ACTION Ablibercept is a recombinant fusion protein that acts as a decoy receptor for the ligands, vascular endothelial growth factor-A (VEGF-A) and placental growth factor (PIGF). It prevents these ligands to binding to endothelial receptors, VEGFR-1 and VEGFR-2, to suppress neovascularization and decrease vascular permeability. This ultimately will slow vision loss or the progression of metastatic colorectal cancer.
ABSORPTION In patients with wet AMD and CRVO, the mean peak plasma concentration (Cmax) was 0.02 mcg/mL and 0.05 mcg/mL respectively. These concentrations were reached in 1 to 3 days. Aflibercept did not accumulate when administered as repeated doses intravitreally every 4 weeks. VOLUME OF DISTRIBUTION After intravenous injection of aflibercept, the volume of distribution is 6 L Metabolism Because aflibercept is a protein, it is expected to be broken down via proteolysis into smaller peptides and amino acids. The cytochrome P450 enzyme system is not involved in the metabolism of aflibercept.
Intravitreal HALF-LIFE = 7.13 days in humans Terminal elimination half-life of free aflibercept in plasma was 5 to 6 days after IV injection of 2 - 4 mg/kg dose. CLEARANCE When cancer patients were given 2-9 mg/kg every 2 or 3 week; 1 hour IV infusion of aflibercept the typical estimated clearances were as follows: CL of free aflibercept (CLf) = 0.88 L/day; CL of bound aflibercept (CLf) = 0.19 L/day; Patients clear free aflibercept faster if they had low albumin or high alkaline phosphatase levels. TOXICITY For all intravitreal VEGF inhibitors, there is increased risk of stroke and myocardial infarction. An increase in intraocular pressure may also occur. When used intravenously, most common adverse reactions were leukopenia, diarrhea, neutropenia, proteinuria, AST increased, stomatitis, fatigue, thrombocytopenia, ALT increased, hypertension, weight decreased, decreased appetite, epistaxis, abdominal pain, dysphonia, serum creatinine increased, and headache.
AFLIBERCEPT EYLEA (for macular degeneration) Intravitreal inj. Zaltrap ((ziv-aflibercept: for colorectal cancer) IV infusion EYLEA (aflibercept) is a recombinant fusion protein consisting of portions of human VEGF receptors 1 and 2 extracellular domains fused to the Fc portion of human IgG1 formulated as an iso-osmotic solution for intravitreal administration. Aflibercept is a dimeric glycoprotein with a protein molecular weight of 97 kilodaltons (kDa) and contains glycosylation, constituting an additional 15% of the total molecular mass, resulting in a total molecular weight of 115 kDa. Aflibercept is produced in recombinant Chinese hamster ovary (CHO) cells. Zaltrap (ziv-aflibercept) is a recombinant fusion protein consisting of Vascular Endothelial Growth Factor fused to the Fc portion of human IgG. Zaltrap injection for intravenous infusion is indicated for patients with metastatic colorectal cancer (mCRC).
Derived from recombinant CHO cells DOSAGE (Eyela): 2 mg (0.05 mL or 50 microliters) administered by intravitreal injection every 4 weeks (monthly) for the first 12 weeks (3 months), followed by 2 mg (0.05 mL) via intravitreal injection once every 8 weeks (2 months). Although EYLEA may be dosed as frequently as 2 mg every 4 weeks (monthly), additional efficacy was not demonstrated when EYLEA was dosed every 4 weeks compared to every 8 weeks DOSAGE (Zaltrap): 4 mg per kg as an intravenous (IV) infusion over 1 hour every two weeks. Administer ZALTRAP prior to any component of the FOLFIRI regimen on the day of treatment VIAL DESCRIPTION: EYLEA is a sterile, clear, and colorless to pale yellow solution. EYLEA is supplied as a preservative-free, sterile, aqueous solution in a single-use, glass vial designed to deliver 0.05 mL (50 microliters) of EYLEA (40 mg/mL in 10 mM sodium phosphate, 40 mM sodium chloride, 0.03% polysorbate 20, and 5% sucrose, pH 6.2).
VIAL DESCRIPTION Zaltrap is available in single-use vials in two strengths: 100 mg/4 mL (25 mg/mL) and 200 mg/8 mL (25 mg/mL). Zaltrap is dosed as 4 mg/kg as an intravenous infusion over 1 hour, every 2 weeks. Serious side effects include hemorrhage, GI perforation and compromised wound healing. Based on animal data, Zaltrap may cause fetal harm. Zaltrap should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. It is not known whether Zaltrap is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious side effects in nursing infants, a decision should be made as to whether to discontinue nursing or to discontinue the drugs, taking into account the important of the drug to the mother. The safety and effectiveness of Zaltrap has not been examined in the pediatric population.
ADVERSE REACTION: Eyela: Hives; difficult breathing; swelling of your face, lips, tongue, or throat. Zaltrap: Severe and sometimes fatal hemorrhage, including gastrointestinal (GI) hemorrhage, has been reported in the patients who have received ZALTRAP in combination with FOLFIRI. Monitor patients for signs and symptoms of GI bleeding and other severe bleeding. Do not administer ZALTRAP to patients with severe hemorrhage. GI perforation including fatal GI perforation can occur in patients receiving ZALTRAP. Discontinue ZALTRAP therapy in patients who experience GI perforation. DOSAGE 2 mg (0.05 mL or 50 microliters) administered by intravitreal injection every 4 weeks (monthly) for the first 12 weeks (3 months), followed by 2 mg (0.05 mL) via intravitreal injection once every 8 weeks (2 months). Although EYLEA may be dosed as frequently as 2 mg every 4 weeks (monthly), additional efficacy was not demonstrated when EYLEA was dosed every 4 weeks compared to every 8 weeks
PROTEIN SEQUENCE FOR AFLIBERCEPT SDTGRPFVEMYSEIPEIIHMTEGRELVIPCRVTSPNITVTLKKFPLDTLIPDGKRIIWDSR KGFIISNATYKEIGLLTCEATVNGHLYKTNYLTHRQTNTIIDVVLSPSHGIELSVGEKLVLN CTARTELNVGIDFNWEYPSSKHQHKKLVNRDLKTQSGSEMKKFLSTLTIDGVTRSDQ GLYTCAASSGLMTKKNSTFVRVHEKDKTHTCPPCPAPELLGGPSVFLFPPKPKDTLMI SRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKPREEQYNSTYRVVSVLTVL HQDWLNGKEYKCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSRDELTKNQVSLTC LVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFS CSVMHEALHNHYTQKSLSLSPG
PATENTS Country United States United States United States United States United States United States Patent Number 7306799 7531173 7374758 7608261 7070959 7374757 Approved 2005-03-25 2009-05-12 2008-05-20 2009-10-27 2006-07-04 2008-05-20 Expires (estimated) 2020-05-23 2026-02-02 2020-05-23 2027-06-14 2020-05-23 2020-05-23
REFERENCES Eyela http://www.ncbi.nlm.nih.gov/pubmed/25200895 http://www.ncbi.nlm.nih.gov/pubmed/24740170 http://www.ncbi.nlm.nih.gov/pubmed/22354219 http://www.ncbi.nlm.nih.gov/pubmed/23461161 Zaltrap http://www.ncbi.nlm.nih.gov/pubmed/25395483 http://www.ncbi.nlm.nih.gov/pubmed/25394166 http://www.ncbi.nlm.nih.gov/pubmed/25393440 http://www.ncbi.nlm.nih.gov/pubmed/25378900 http://www.ncbi.nlm.nih.gov/pubmed/25378873