AGATE-II Study: Ombitasvir/Paritaprevir/Ritonavir + Ribavirin in Egyptian Patients
Study on the effectiveness of ombitasvir/paritaprevir/ritonavir + ribavirin in genotype 4 Egyptian patients with or without cirrhosis. The study includes treatment regimens, baseline characteristics, SVR12 rates, and reasons for not achieving SVR12.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
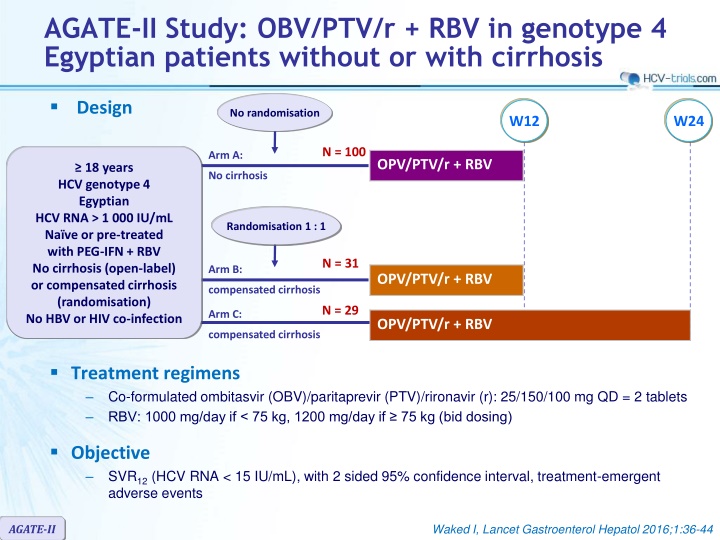
AGATE-II Study: OBV/PTV/r + RBV in genotype 4 Egyptian patients without or with cirrhosis Design No randomisation W12 W24 N = 100 Arm A: OPV/PTV/r + RBV 18 years HCV genotype 4 Egyptian HCV RNA > 1 000 IU/mL Na ve or pre-treated with PEG-IFN + RBV No cirrhosis (open-label) or compensated cirrhosis (randomisation) No HBV or HIV co-infection No cirrhosis Randomisation 1 : 1 N = 31 Arm B: OPV/PTV/r + RBV compensated cirrhosis N = 29 Arm C: OPV/PTV/r + RBV compensated cirrhosis Treatment regimens Co-formulated ombitasvir (OBV)/paritaprevir (PTV)/rironavir (r): 25/150/100 mg QD = 2 tablets RBV: 1000 mg/day if < 75 kg, 1200 mg/day if 75 kg (bid dosing) Objective SVR12(HCV RNA < 15 IU/mL), with 2 sided 95% confidence interval, treatment-emergent adverse events AGATE-II Waked I, Lancet Gastroenterol Hepatol 2016;1:36-44
AGATE-II Study: OBV/PTV/r + RBV in genotype 4 Egyptian patients without or with cirrhosis Baseline characteristics No cirrhosis 12W N = 100 49 30% 29.1 6.01 44 / 40 /16 Cirrhosis 12W N = 31 57 6% 29.3 6.02 42 / 42 / 16 Cirrhosis 24W N = 29 Mean age, years Female Mean BMI, kg/m2 Mean HCV RNA, log10IU/mL Subgenotye: 4a / 4c-d / other, not det., % 56 24% 31.0 5.97 34 / 38 / 28 Fibrosis stage (%) : F0-F1 / F2 / F3 / F4 68 / 11 / 19 / 2 0 / 0 / 3 / 97 0 / 0 / 0 /100 Treatment-na ve, % Treatment-experienced, % Null responder Partial responder Relapser Mean platelet count (x 109/l) Mean albumin (g/L) History of diabetes, % 49 48 52 33 8 10 229 29 6 16 156 24 7 17 138 44.7 + 3.0 15 41.8 + 4.4 29 40.3 + 5.3 24 AGATE-II Waked I, EASL 2016, Abs. SAT-166, J Hepatol 2016;64:S772, Waked I, Lancet Gastroenterol Hepatol 2016;1:36-44
AGATE-II Study: OBV/PTV/r + RBV in genotype 4 Egyptian patients without or with cirrhosis SVR12rates by treatment arm, % (95% CI) % SVR12by baseline fibrosis stage (12 week treatment arms), % (95% CI) % 97 94 93 (84-99) 100 (88-97) (78-98) 95 94 (89-98) (80-98) 100 80 80 60 60 40 40 20 20 94/100 30/31 27/29 94/99 30/32 0 0 Arm A No-cirrhosis 12 weeks Arm B Cirrhosis 12 weeks Arm C Cirrhosis 24 weeks < F3 = F4 Baseline fibrosis stage Sensitivity analysis : SVR12excluding patients with premature discontinuation of study drug with no on-treatment failure or with missing follow-up data in SVR12window: 96% (94/98) for Arm A 97% (30/31) for Arm B 96% (27/28) for Arm C AGATE-II Waked I, Lancet Gastroenterol Hepatol 2016;1:36-44
AGATE-II Study: OBV/PTV/r + RBV in genotype 4 Egyptian patients without or with cirrhosis Reasons for not achieving SVR12 No cirrhosis 12 weeks Cirrhosis 12 weeks Cirrhosis 24 weeks Non-response 2/100 (2.0%) 1/31 (3.2%) 1/29 (3.4%) On-treatment virologic failure 1 (1.0%) 1 (3.2%) 1/29 1 (1.0%) Premature study drug discontinuation 0 0 withdrew consent Missing SVR12data 1/100 0 1 Relapse by W12 post-treatment 3/98 (3.1%) * 0 0 * Includes 1 patient with compensated cirrhosis miscategorized as non-cirrhotic and assigned in Arm A AGATE-II Waked I, Lancet Gastroenterol Hepatol 2016;1:36-44
AGATE-II Study: OBV/PTV/r + RBV in genotype 4 Egyptian patients without or with cirrhosis Treatment-emergent adverse events Cirrhosis 24W N = 29 25 (86%) 0 7 0 No cirrhosis 12W N = 100 80 (80%) 0 2 1 Cirrhosis 12W N = 31 26 (84%) 0 0 0 Any treatment-emergent adverse event Adverse event leading to discontinuation Serious adverse event, % Death, N Adverse events occurring in 10% in either group, % Headache Fatigue Pruritus Dyspepsia Upper abdominal pain Cough Insomnia ALT Grade 2 (> 3 x ULN) AST Grade 2 (> 3 x ULN) Total bilirubin grade 3 (> 3-10 x ULN), % Hemogloblin 8-10 g/dL, N (%) RBV dose reduction, N (%) 41 35 23 17 19 6 9 0 0 2 29 29 13 13 6 12 6 0 0 6 35 38 31 14 10 31 17 0 0 14 7 (7%) 11 (11%) 2 (6%) 4 (13%) 4 (14%) 6 (21%) AGATE-II Waked I, Lancet Gastroenterol Hepatol 2016;1:36-44
AGATE-II Study: OBV/PTV/r + RBV in genotype 4 Egyptian patients without or with cirrhosis Summary High SVR rates were achieved in HCV genotype 4-infected Egyptian patients without cirrhosis or with compensated cirrhosis after 12 weeks of OBV/PTV/r + RBV weeks: SVR12was 94% and 97%, respectively 12 weeks treatment was well tolerated with no treatment discontinuations due to adverse events Prolongation of therapy to 24 weeks does not provide additional benefit in patients with cirrhosis, with more serious adverse events and a higher frequency of hemoglobin decrease AGATE-II Waked I, Lancet Gastroenterol Hepatol 2016;1:36-44