Alcohols and Phenols in Chemistry: Properties and Reactions
Explore the classification, nomenclature, methods of preparation, and chemical properties of alcohols. Learn about unsaturated alcohols, allyl alcohol, and various reactions involving double bonds and hydroxyl groups.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Alcohols and Phenols - I B.Sc. II year Mohanlal Sukhadia University, Udaipur Dr. Tarun Kumar Assistant Professor Department of Chemistry, M. L. Sukhadia University, Udaipur
Alcohols General formula R-OH Alcohols are alkyl derivatives of water or hydroxyl derivatives of hydrocarbons
Classification of Alcohols (i) Classification based upon number of hydroxyl group (ii) Classification based upon nature of carbon atom bearing hydroxyl group
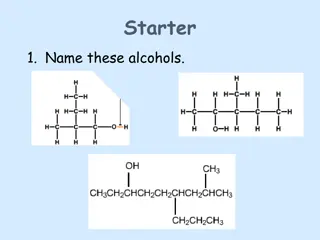
Monohydric Alcohols Nomenclature (i) Trivial system (ii) Derived system (iii) IUPAC system
Monohydric Alcohols Unsaturated alcohols Vinyl alcohols are highly unstable and they quickly tautomerizes to stable keto form acetaldehyde.
Methods of Preparation From alkyl halides
Methods of Preparation Allyl Alcohol 1. From glycerol 2. From propargyl alcohol
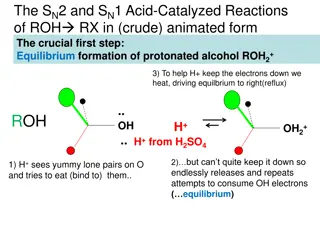
Chemical Properties 1. Reactions of double bond (i) Reduction (ii) From halogen (iii) From halogen acid
Chemical Properties (iv) Hydroxylation (iv) Ozonization
Chemical Properties 2. Reactions of OH group (i) With sodium (ii) With fatty acids (iii) With inorganic acids
Chemical Properties (iv) Oxidation