Alkenes: Properties and Reactions
Explore the world of alkenes in organic chemistry, including the first 4 alkenes, their general formula, reactions with bromine water, combustion properties, and more. Learn about the differences between alkenes and alkanes, their functional groups, and why octane is preferred over octene in car engines. Dive into addition reactions and create a poster comparing alkenes and alkanes.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
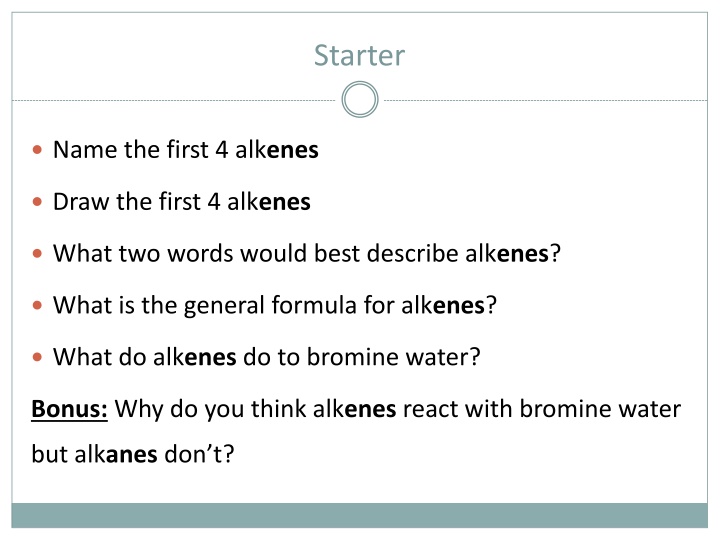
Starter Name the first 4 alkenes Draw the first 4 alkenes What two words would best describe alkenes? What is the general formula for alkenes? What do alkenes do to bromine water? Bonus: Why do you think alkenes react with bromine water but alkanes don t?
Organic Chemistry ALKENES
Covalent Bonding in Alkenes Try and draw the covalent bonding in the first 4 alkenes Only draw the outer shell of electrons Extension: explain how this is different to the covalent bonding in alkanes?
Some New Key Words Define the following terms: Functional Group Homologous Series State the functional group of alkenes How does the successive members of alkenes differ from each other? Bonus: Do you know any other homologous series?
Combustion of Alkenes Burn with a smokier, yellow flame (compared to alkanes) Incomplete combustion Release less energy per mole in combustion than alkanes e.g. of complete C2H4 + 3O2 2CO2 + 2H2O
AfL Explain why someone would chose to use octane in a car engine and not octene? (4 marks) Octene tends to undergo incomplete combustion so produces a smokier flame than octane. This also produces more CO which is poisonous. Octane releases more energy per mole during combustion than octene so is a more efficient fuel.
Research For prep, complete a poster on Alkenes vs. Alkanes Must include: Basic information on both E.g. general formula, functional group etc. Uses for both At least one use with a reason why it is used for that Must mention the specific names of at least two of each 10 minutes now to start some research Please complete BY HAND!
Addition Reactions The C=C in alkenes make them far more reactive than alkanes This is because the C=C is electron dense The double bond can open up and other molecules can react with the carbons, add to the alkene These are called addition reactions
Addition Reactions Halogens How do we know halogens and alkenes undergo addition reactions? Bromine water turning colourless Alkane + Bromine water (no reaction) Alkene + Bromine water (turns colourless) Alkenes will also react with other halogens: Chlorine Bromine Iodine
Addition Reactions Halogens Use your molymods to think of a product What would you name it? Br + Br Bromine Dibromoethane Ethene Colourless
Try Complete the following equations and name the product + Br Br C4H8 + Br2 C2H4 + Cl2 C3H6 + I2
Addition Reactions Hydrogen Alkenes are unsaturated so we can add more hydrogen to make them saturated Hydrogenation 60 C Nickel catalyst + H H Hydrogen Ethene Ethane
Research Why? Why would we want to turn an alkene in an alkane? Why go from unsaturated to saturated? Advantages/Disadvantages Straighten the molecules Increase melting point E.g. to make margarine Enough hydrogen added to make it spreadable straight from the fridge (not too solid)
6 Mark Question In this question you will be assessed on using good English, organising information clearly and using specialist terms where appropriate. Unsaturated fats can be changed into saturated fats by a process of Hydrogenation. Describe the process of Hydrogenation and the reaction, then evaluate the advantages and disadvantages of converting unsaturated fats into saturated.
Peer Marking When you are asked to peer mark you need to: Give them a mark out of 6, from the criteria Give them an effort grade (1-5) Also include a WWW (what went well) This could be that they really understood a certain aspect Was presented well Used lots of key words Etc... And an EBI (even better if) What did they get wrong? What did they miss out? Could there have been more scientifically relevant language used?
Mark Scheme 1-2 marks (D-E) 3-4 marks (C-B) 5-6 marks (A-A*) Simple description of hydrogenation. Good description of hydrogenation. Excellent description of One disadvantage or advantage given. One disadvantage and advantage hydrogenation. One disadvantage Spelling, punctuation and grammar is given. Spelling, punctuation and and advantage given, and not very good. grammar is okay. evaluated. No mistakes in spelling, punctuation and grammar. Key Points for Hydrogenation Addition of hydrogen across the double bond Replaces C=C for C-C (unsaturated to saturated) Uses a nickel catalyst at 60 C This causes the melting point of the fat to increase, therefore they become solid at room temperature Disadvantages Saturated fats are not a healthy option, as the molecules pack closely together, making it harder for the body to break down Advantages As now solid, can be used as a spread (margarine) More useful
Starter Explain, as fully as possible, why alkenes turn bromine water colourless
Addition Reactions Water (Steam) Ethanol (and other alcohols) can be made from the hydration of ethene (and other alkenes) Hydration is the addition of water Concentrated phosphoric acid catalyst High temperature and pressure + Ethanol Water Ethene
Addition Reactions Water (Steam) The reaction is reversible so ethanol can break back down into steam and ethene Unreacted ethene and steam are recycled over the catalyst
Discuss with your Partner What is the problem when you have the following reaction: + Why wasn t this a problem when reacting with Br2 and H2? Do you think there is a problem for the below reaction? +
All Addition Reactions In all addition reactions, only one molecule of halogen, hydrogen or water is needed Only one double bond to open up Halogens, e.g. Br2 One bromine from the bromine molecule bonds to one carbon from the double bond, then the other Br bonds to the other carbon from the double bond Hydrogen, H2 Same concept, but with H-H Water, H2O H bonds to one of the carbons, then OH bonds to the other How many hydrogen molecules would I need if I had two double bonds? Why?
Naming Halogens Dibromo (bromine) Dichloro (chlorine) Diiodo (iodine) Hydrogenation ene to ane Ethene to ethane Hydration Will look at naming alcohols in a few lessons time!
Mechanisms A mechanism shows how a specific reaction is taking place In the below diagram, you can see how the double bond is opening up to initially bond to one Br Then the other Br bonds to the carbon with only 3 bonds Forming dibromoethane
Quiz Number 1-9 in your books. A* = 9 out of 9 A = 7 out of 9 B = 5 out of 9 C = 3 out of 9 D = 2 out of 9 E = 1 out of 9 U = 0 out of 9 You will have 20 seconds to answer the question, the slide will change automatically. There is no going back to previous questions.
Question 1 E Grade The addition of water is called: a) Halogenation b) Hydration c) Hydrogenation
Question 2 D grade What are the benefits of hydrogenation? a) Increases the melting point b) Makes fats healthier c) Adds more hydrogen
Question 3 D Grade Is this molecule saturated or unsaturated ?
Question 4 C Grade Can this molecule undergo addition reactions, why?
Question 5 B Grade What is the definition of a functional group? a) Family of organic molecules with the same functional group b) Gives a family of organic molecules their characteristic reactions c) Compound consisting of just hydrogen and carbon Bonus: can you give the key word for the other two definitions?
Question 6 B Grade Draw the structure of the molecule if bromine water were added to the molecule below
Question 7 A Grade Draw the structure of the molecule if bromine water were added to the molecule below
Question 8 A Grade Draw the two structures if water were added to the molecule below
Question 9 A* Grade Why do two different structures form when it reacts with water but not when it reacts with bromine?