Amino Acid Metabolism and Anabolism in Biology
Explore the intricate processes of amino acid metabolism and anabolism in biological systems, including the categorization of amino acids, essential vs. non-essential amino acids, and the synthesis of proteins and DNA. Gain insights into the pathways that build larger molecules and the vital roles they play in cellular functions and growth.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Dr. C. Mercy, Assistant professor, PG Department of zoology, Sarah Tucker College, Tirunelveli.
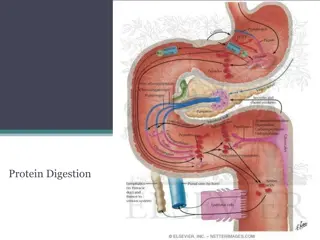
GENERAL PATHWAY OF AMINO ACID METABOLISM, CATABOLISM OF TYROSINE, TRYPTOPHAN, GLYSINE AND PHENYLALANINE
Amino acid metabolism Amino acids are categorized into two types - non-essential amino acids (can be synthesized by the body) and essential amino acids which cannot, and have to be provided from the diet. The non-essential amino acids are glycine, alanine, serine, asparagine, aspartic acid, glutamine, glutamic acid, proline, cysteine, tyrosine and arginine. The essential amino acids include valine, leucine, isoleucine, phenylalanine, tryptophan, methionine, threonine, lysine and histidine. The amino acid pool comes from protein degradation in the gastro- intestinal tract, intracellular protein degradation and de novo synthesis and is used in protein synthesis and metabolism.
Anabolism Definition Anabolism collectively refers to all the processes of chemical reactions that build larger molecules out of smaller molecules or atoms; these processes are also known as anabolic processes or anabolic pathways. The opposite of anabolism is catabolism, the set of processes that breaks down larger molecules into smaller ones. Anabolism and catabolism are the two types of metabolic pathways. Metabolic pathways are series of chemical reactions that take place in the cell. Anabolic pathways use energy, while catabolic pathways release energy.
Examples of Anabolic Processes Protein Synthesis Proteins are macromolecules that carry out cellular activities encoded by an organism s genes. They have many different functions in the body, including DNA replication, aiding chemical reactions (as enzymes), transporting materials in the cell, cell growth and signaling, and providing physical structure. Each cell in the human body contains about 1 to 3 billion proteins. Proteins are synthesized from smaller molecules called amino acids in a cell s ribosomes. Each protein is a chain of a specific sequence of amino acids. Since proteins are larger molecules put together from smaller ones, the process of protein synthesis is anabolic.
DNA Synthesis Deoxyribonucleic acid, or DNA, is an organism s genetic material. It is macromolecule made up of smaller molecules called nucleic acids, which are themselves made up of a nucleotide base attached to a deoxyribose sugar and a phosphate molecule. DNA synthesis is an anabolic process that takes place in a cell s nucleus just before the cell divides. It involves unzipping the double strands of DNA and attaching new matching nucleotides to each half of the strand that has been unzipped, forming two new strands that each contain half of the old DNA strand.
Growth of Bones and Muscles On a larger scale, the growth of body parts such as bones and muscles is anabolic. Bone growth, or ossification, occurs when bone is formed from cells called osteoclasts. It is then mineralized through cells called osteoblasts. This process is also anabolic; during mineralization, osteoblasts produce calcium phosphate crystals that are incorporated into the bone s structure, making the bones hard and sturdy. Muscle growth, also called muscle hypertrophy, occurs when the cells of skeletal muscles, called myocytes, increase in size. It occurs through strength training exercises such as lifting weights. Factors like sex, age, and diet all affect hypertrophy. During hypertrophy, there is increased protein synthesis of actin and myosin, and the volume of sarcoplasmic fluid in the myocyte increases.
catabilism DEAMINATION OF AMINO ACIDS Introduction: Deamination is also an oxidative reaction that occurs under aerobic conditions in all tissues but especially the liver. During oxidative deamination, an amino acid is converted into the corresponding keto acid by the removal of the amine functional group as ammonia and the amine functional group is replaced by the ketone group. The ammonia eventually goes into the urea cycle. Oxidative deamination occurs primarily on glutamic acid because glutamic acid was the end product of many transamination reactions. The glutamate dehydrogenase is allosterically controlled by ATP and ADP. ATP acts as an inhibitor whereas ADP is an activator.
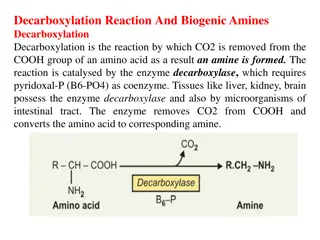
Decarboxylation Decarboxylation is a chemical reaction that releases carbon dioxide (CO2). Usually, decarboxylation refers to a reaction of carboxylic acids, removing a carbon atom fromchain. The reverse process, which is the first chemical step in photosynthesis, is called carbonation, the addition of CO2 to a compound. Enzymes that catalyze decarboxylationcalled decarboxylases or, the more formal term, carboxy-lyases urea cycle The urea cycle is a cycle of biochemical reactions occurring in many animals that produces urea from ammonia. This cycle was the first metabolic cycle discovered five years the discovery of the TCA cycle. In mammals, the urea cycle takes place primarily in the liver, and to a leaser extend in the kidney
The urea cycle consists if five reactions two mitochondrial and three cytosolic. The cycle converts two amino groups, one from NH4+ and one from Asp, and a carbon atom from HCO3-, to the relatively nontoxic excreation product urea at the cost of four high energy phosphate bonds. Ornithine is the carrier of these carbon and nitrogen atoms.
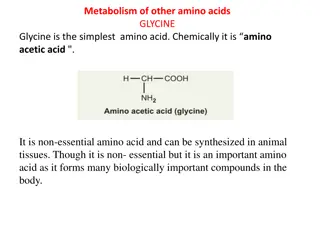
Glycine Catabolism Glycine is classified as a glucogenic amino acid, since it can be converted to serine by serine hydroxymethyltransferase, and serine can be converted back to the glycolytic intermediate, 3-phosphoglycerate or to pyruvate by serine/threonine dehydratase, although the latter reaction (serine to pyruvate) appears not be occur in human tissues. Nevertheless, the main glycine catabolic pathway leads to the production of CO2, ammonia, and one equivalent of N5,N10-methyleneTHF by the mitochondrial enzyme, glycine dehydrogenase (decarboxylating) which is also called the glycine cleavage complex, GCC. The GCC is composed of four mitochondrial proteins encoded by four genes. The protein components of the GCC are the actual glycine dehydrogenase subunit (identified as the P subunit: pyridoxal phosphate-dependent), a lipoic acid-containing subunit (the H subunit), a tetrahydrofolate-requiring enzyme called aminomethyltransferase (the T subunit), and dihydrolipoamide dehydrogenase (the L subunit).
Phenylalanine and Tyrosine Catabolism Phenylalanine normally has only two fates: incorporation into polypeptide chains, and hydroxylation to tyrosine via the tetrahydrobiopterin-requiring phenylalanine hydroxylase (PAH) reaction. Thus, phenylalanine catabolism always ensues in the pathway of tyrosine biosynthesis followed by tyrosine catabolism. Tyrosine is equally important for protein biosynthesis as well as an intermediate in the biosynthesis of the catecholamines: dopamine, norepinephrine and epinephrine.
Tryptophan Catabolism Catabolism of tryptophan in humans results in the formation of acetoacetate. The first enzyme of the tryptophan catabolic pathway opens the indole ring. This enzyme is tryptophan 2,3-dioxygenase which is encoded by the TDO2 gene located on chromosome 4q31 q32 and is composed of 12 exons that encode a protein of 406 amino acids.