Amino Acids: Structure, Nomenclature, and Classification
Explore the world of amino acids, organic compounds crucial for life, containing amine and carboxylic groups. Learn about their structure, nomenclature, and classification into essential/non-essential, neutral, acidic, and basic types, along with variations in side chains.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
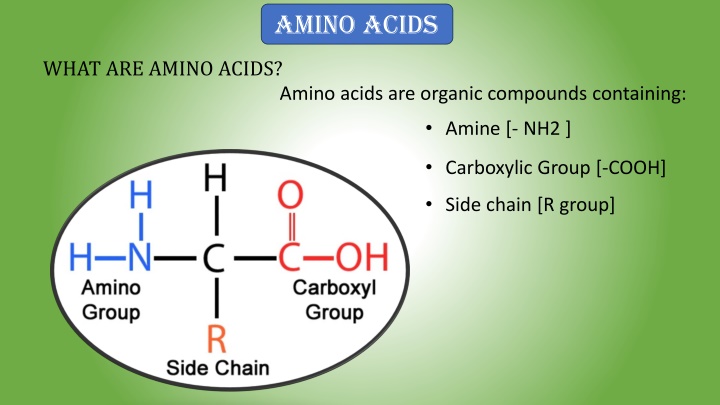
AMINO ACIDS WHAT ARE AMINO ACIDS? Amino acids are organic compounds containing: Amine [- NH2 ] Carboxylic Group [-COOH] Side chain [R group]
The major key elements if amino acids are carbon, hydrogen, nitrogen, oxygen. About 500 amino acids are known (though only 20 appear in the genetic code) and can be classified in many ways Depending upon the position of the amino group with respect to the carboxyl group, Amino acids are classed as -, -, - or - amino acids. The carbon atom next to the carboxyl group is regarded as -carbon atom. Nearly all the naturally occurring amino acids are -amino acids that occur as constituents of proteins and have an amino group and carboxyl group attached to the same carbon atom.
NOMENCLATURE OF AMINO ACIDS: Although amino acids can be named according to IUPAC system, they are generally known by their common names. For Example, aminoethanoic acid, NH2CH2COOH is named Glycine. Each amino acid has been assigned an abbreviation which generally consist of the first three letters of the common name. E.g. Glycine is abbreviated as Gly, Alanine is abbreviated as Ala.
STRUCTURE OF AMINO ACIDS(Zwitter Ions): Amino acids contain both a basic group and an acidic group. They are amphoteric compounds. According to the structure of amino acids, the acidic properties should be due to the carboxylic group and the basic properties are due to the amino group. However, Modern research showed that it is not correct. + group and basic properties are due to ??? . The acidic properties are due to ??3 This is because the proton from the carboxyl group shifts to the amino group to produce dipolar ion. Such ion is called the inner salt or the Zwitter ion.
CLASSIFICATION OF -AMINO ACIDS: a) Essential and non-essential amino acids: Human body can synthesize 10 out of 20 amino acids found in proteins and we do not require them in our diet. These are called non-essential amino acids. amino acids are called essential amino acids. To make up the deficiency of the rest ten, these must be supplied in the diet. These b) Neutral, Acidic and Basic amino acids: Neutral amino acids: Such amino acids contain one ??2 group and one COOH group. E.g. Glycine, Alanine, Serine Basic amino acids: It contains more ??2 group compared to COOH groups. E.g. Lysine, Histidine iii) Acidic amino acids: It contains more COOH groups compared to ??2 group. E.g. Aspartic acid, Glutamic acid. i) ii)
c) Amino acids with different side chains: c) Amino acids with different side chains: i) With non-polar side chain: E.g. Valine, Isoleucine ii) With polar (unionised) side chain: E.g. Serine, Cysteine, Asparagine iii) With acidic side chain: E.g. Aspartic acid and Glutamic acid. iv) With basic side chain: E.g. Lysine and Histidine STEREO CHEMISTRY OF -AMINO ACIDS: All -amino acids, except glycine have chiral carbon atom and have two optically active isomers. All naturally occurring amino acids have L-configuration i.e. they have ??2 group on the left. In terms of R and S notation, they have S-Configuration
Acid Acid- -Base Behaviour of Amino Acids Base Behaviour of Amino Acids a) Basic Character: b) Acidic Character: Q. Why low values of ??? and ??? for amino acids? Due to the dipolar nature of amino acids. In -amino acids, the acidic group is NH3 instead of COOH group in carboxylic acids. Similarly, the kb values of -amino acids are very low because the basic group is COO- instead of HN2 group in aliphatic amines. The Basic unit of amino acids is ??? and thus, they form salts with HCl. +and thus, they form salts with NaOH. The Acidic unit of amino acids is ??3 Q. Amino acid glycine (NH2 CH2 COOH) exists as a zwitterion in aqueous solution. The Ka and Kb values of glycine are 1.6 10 10 (??? =9.8) and 2.5 10 12(??? =11.6) respectively. The Ka and Kb values are for zwitterion of amino acid with the following structure [NH3 CH2 ??? ]. What is the Kb for NH2 group in glycine?
Effect of pH on the structure of -amino acids The structure of amino acids depends upon the pH of the medium containing amino acid. In the acidic medium, the dipolar ion gets converted to a cation or weaker acid. ??3 ?? ? ??? + ?3?+ ??3 ?? ? ???? +?2? But when the solution of amino acid is made alkaline, then the stronger base abstracts a proton from the dipolar ion and forms a weaker base. ??3 ?? ? ??? + ?? ??2 ?? ? ??? +?2?
THE ISOELECTRIC POINT: The isoelectric point (pI) of an amino acid is the pH at which there is no net migration of the amino acid under the influence of applied field. In other words, it is the pH at which the amount of positive charge on an amino acid exactly balances the amount of negative charge. pI = pH at which there is no net charge It may be noted that, i) At a pH lower than pI, there will be net migration of amino acid towards cathode as the amino acid mainly exists as cation. ii) At a pH higher than pI, there will be net migration of amino acid towards anode as the amino acid mainly exists as anion.
Determining the pI of an Amino Acid without an Ionizable Side Chain The pI of an amino acid that does not have an ionizable side chain such as alanine is midway between its two pKa values. This is because at pH = 2.34 half of the molecules have a negatively charged carboxyl group and half have an uncharged carboxyl group, whereas at pH = 9.69 half of the molecules have a positively charged amino group and half have an uncharged amino group. As the pH increases from 2.34, the carboxyl group of more molecules becomes negatively charged; as the pH decreases from 9.69, the amino group of more molecules becomes positively charged. Therefore, the number of negatively charged groups equals the number of positively charged groups at the intersection (average) of the two pKa values.
Determining the pI of an Amino Acid with an Ionizable Side Chain The pI of most amino acids that have an ionizable side chain is the average of the pKa values of the similarly ionizing groups (either positively charged groups ionizing to uncharged groups or uncharged groups ionizing to negatively charged groups). For example, the pI of lysine is the average of the pKa values of the two groups that are positively charged in their acidic form and uncharged in their basic form. The pI of glutamic acid is the average of the pKa values of the two groups that are uncharged in their acidic form and negatively charged in their basic form.
Electrophoresis: Electrophoresis is a separation technique based on the movement of charged ions under the influence of an electrical field. This technique is primarily used for the separation of amino acids and peptides on the basis of their charge. Electrophoresis separates amino acids on the basis of their pI values. In Paper electrophoresis, a strip of paper or cellulose acetate is used as a solid support. A mixture of amino acids is placed in the form of a spot in the centre of strip. The strip is then soaked with an aqueous buffer of a particular pH which depends upon the pI of amino acids in the mixture. The two ends of the paper are then dipped into the buffer solution having electrodes. Now electric field is applied.
The following changes are observed: i. The amino acids with low isoelectric point compared to the pH of the buffer solution start moving towards anode since they exist mainly in the anionic form ii. The amino acids with higher isoelectric point compared to the pH of the buffer solution start moving towards cathode because they mainly exist in cationic form. iii. The amino acids with isoelectric point equal to or comparable to the buffer solution do not migrate from the origin.
SYNTHESIS OF AMINO ACIDS: HVZ Reaction Followed by Reaction with Ammonia One of the oldest methods used to synthesize an amino acid is to employ an HVZ reaction to replace an -hydrogen of a carboxylic acid with a bromine. The resulting -bromocarboxylic acid can then undergo an SN2 reaction with ammonia to form the amino acid.
Reductive Amination Amino acids can also be synthesized by reductive amination of an -keto acid. The keto acid is treated with ammonia to form an imine. The imine is catalytically reduced to amino acid.
N-Phthalimidomalonic Ester Synthesis Amino acids can be synthesized with better yields than those obtained by the previous two methods via an N-phthalimidomalonic ester synthesis, a method that combines the malonic ester synthesis and the Gabriel synthesis. -Bromomalonic ester and potassium phthalimide undergo an SN2 reaction. A proton is easily removed from the a-carbon of N-phthalimidomalonic ester because it is flanked by two carbonyl groups. The resulting carbanion undergoes an SN2 reaction with an alkyl halide. Heating in an acidic aqueous solution hydrolyses the two esters and the two amide bonds and decarboxylates the 3-oxocarboxylic acid
Strecker Synthesis In the Strecker synthesis, an aldehyde reacts with ammonia to form an imine. A nucleophilic addition reaction with cyanide ion forms an intermediate, which, when hydrolyzed, forms the amino acid
PROPERTIES OF -AMINO ACIDS a) Physical Properties: 1. All Amino acids are crystalline solids with high melting and boiling points. 2. These are soluble in polar solvents like water but insoluble in organic solvents like benzene. 3. They have high values of the dipole moments and exist as dipolar molecules. 4. Except glycine, all are amino acids are optically active. b) Chemical Properties: The Amino acids show characteristic reactions of ??2 group as well as COOH group. Reaction of COOH group are carried in the acidic medium. Similarly, reactions of amino group are carried in the basic medium.
A. Reactions due to amino Group: i) Basic character: Amino acids reacts with strong acids like HCl to form salts ?3?+ ??2??? + ??? (?3?+ ??2????)?? ii) Alkylation: In basic medium, it forms N-alkyl amino acid anion when treated with alkyl halide. With excess of alkyl halide, an internal quarternary ammonium salt is formed. iii) Acylation: When acetyl chloride, amino acids undergo acylation.
iv) Reaction with Nitrous acids: Amino acids with primary amino group react with nitrous acid to form hydroxy acids. v) Reaction with 2,4-Dinitrofluoro benzene(Sanger s reagent): In the basic medium, they react with 2,4-Dinitrofluorobenzene to form 2,4-Dinitrophenyl amino acids.
B. Reactions due to Carboxyl group: i) Reaction with bases: Amino acids react with strong bases like NaOH, KOH to form salts.
