Analysis of Cardiovascular Safety with Fremanezumab in Migraine Patients
Explore the cardiovascular safety of Fremanezumab in patients with migraine, presented at the AHS Virtual Annual Scientific Meeting. The study highlights the well-tolerated nature of Fremanezumab in patients with cardiovascular risk factors, emphasizing its efficacy and low incidence of adverse events. Learn about the importance of understanding medications targeting the CGRP pathway for migraine treatment.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
SLIDE KIT THIS ACTIVITY IS SUPPORTED BY AN EDUCATIONAL GRANT FROM LILLY For further information concerning Lilly grant funding visit www.lillygrantoffice.com www.lillygrantoffice.com
SLIDE KIT Pooled Analysis of Cardiovascular Safety with Pooled Analysis of Cardiovascular Safety with Fremanezumab Treatment in Patients with Migraine by Number of Treatment in Patients with Migraine by Number of Cardiovascular or Cerebrovascular Risk Factors Cardiovascular or Cerebrovascular Risk Factors Fremanezumab Presented by Tim P. J rgens, MD, PhD Head of Headache Center North-East Dept. of Neurology, Headache Center North-East, University Medical Center of Rostock Rostock, Mecklenburg-Vorpommern, Germany Developed by Infomedica Medical Education& Information AHS Virtual Annual Scientific Meeting content is made available to an international audience through a licensing agreement between Infomedica and the American Headache Society. Infomedica is an independent medical education provider that delivers medical information to healthcare professionals through conference coverage and online educational programs and activities.
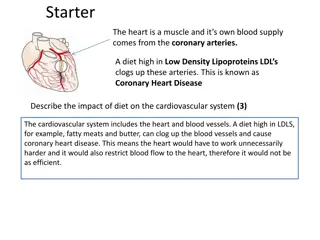
A M E R I C A N H E A D A C H E S O C I E T Y V I R T U A L A N N U A L S C I E N T I F I C M E E T I N G O F F I C I A L H I G H L I G H T S Key messages It is critically important to understand the cardiovascular safety of medications targeting the calcitonin gene-related peptide (CGRP) pathway because of CGRP s known vascular and cardiac effects.1 Fremanezumab treatment was well-tolerated in migraine patients with 2 cardiovascular (CV) risk factors. Fremanezumab did not increase the risk of CV adverse events compared to placebo, even in those with difficult-to-treat migraine. CV adverse events were infrequent, regardless of the number of CV risk factors and CV medical history. DEVELOPED BY
A M E R I C A N H E A D A C H E S O C I E T Y V I R T U A L A N N U A L S C I E N T I F I C M E E T I N G O F F I C I A L H I G H L I G H T S Background What do we already know about this topic? CGRP receptors are located in the central and peripheral nervous system, blood vessels and the heart.2 CGRP is a potent vasodilator and a suspected safeguard during cerebral and cardiac ischemia. Continuous blockage of CGRP with CGRP antagonists raise concerns that transient ischemic events could lead to major infarcts.1 Fremanezumab is a fully-humanized monoclonal antibody (mAb) that selectively targets CGRP, has been studied in three phase 3 trials and has proven efficacy as a preventative treatment of adult migraine.3,4,5 DEVELOPED BY
A M E R I C A N H E A D A C H E S O C I E T Y V I R T U A L A N N U A L S C I E N T I F I C M E E T I N G O F F I C I A L H I G H L I G H T S Background How was this study conducted? Pooled analysis of 3 randomized, double-blind, 12-week, phase 3 trials on patients with episodic migraine, chronic migraine and those with episodic or chronic migraine with prior inadequate response to preventative medications (N=2842). Across the three trials, patients had been randomized 1:1:1 to receive quarterly or monthly subcutaneous injections of fremanezumab 675 mg, fremanezumab 225 mg or placebo. Co-primary end points were the number of adverse events considering CV risk factors at baseline, with or without CV medical history. Of note, patients were excluded if they had clinically significant CV disease, vascular ischemia (myocardial, vascular, or cerebral), TIAs, or DVTs. DEVELOPED BY
A M E R I C A N H E A D A C H E S O C I E T Y V I R T U A L A N N U A L S C I E N T I F I C M E E T I N G O F F I C I A L H I G H L I G H T S Findings What does this study add? CV adverse events noted were hypertension, hypotension, palpitations, supraventricular tachycardia, QT prolongation, and Raynaud s. CV adverse events were low in participants with or without CV history. CV adverse events did not increase with the number of CV risk factors at baseline. No CV adverse events were seen in participants with at least four CV risk factors at baseline. The most common CV risk factors at baseline were hypertension, obesity and use of hormonal birth control pills. DEVELOPED BY
A M E R I C A N H E A D A C H E S O C I E T Y V I R T U A L A N N U A L S C I E N T I F I C M E E T I N G O F F I C I A L H I G H L I G H T S Perspectives How does this study impact clinical practice? Fremanezumab treatment is well-tolerated in migraine patients and does not increase the risk of CV adverse events , even in those with increased CV risk factors. Further studies are needed on the use of fremanezumab treatment in patients with serious CV diseases. DEVELOPED BY
A M E R I C A N H E A D A C H E S O C I E T Y V I R T U A L A N N U A L S C I E N T I F I C M E E T I N G O F F I C I A L H I G H L I G H T S References 1. MaassenVanDenBrink A, Meijer J, Villal n CM, Ferrari MD. Wiping Out CGRP: Potential Cardiovascular Risks. Trends in Pharmacological Sciences 2016;37:779-788. 2. Favoni V, Giani L, Al-Hassany L, et al. CGRP and migraine from a cardiovascular point of view: what do we expect from blocking CGRP? The Journal of Headache and Pain 2019;20(1):27. 3. Silberstein SD, Dodick DW, Bigal ME, et al. Fremanezumab for the Preventive Treatment of Chronic Migraine. The New England Journal of Medicine 2017;377:2113-2122. 4. Dodick DW, Silberstein SD, Bigal ME, et al. Effect of Fremanezumab Compared With Placebo for Prevention of Episodic Migraine: A Randomized Clinical Trial. JAMA 2018;319:1999-2008. 5. Ferrari MD, Diener HC, Ning X, et al. Fremanezumab versus placebo for migraine prevention in patients with documented failure to up to four migraine preventive medication classes (FOCUS): a randomised, double-blind, placebo-controlled, phase 3b trial. Lancet 2019;394:1030-1040. DEVELOPED BY