Analytical Chemistry Lab Standardization of Sodium Hydroxide
"Learn about the standardization process of sodium hydroxide in the Analytical Chemistry lab using indicators and titration curves. Explore the importance of indicators like phenolphthalein in neutralization reactions between acids and bases."
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
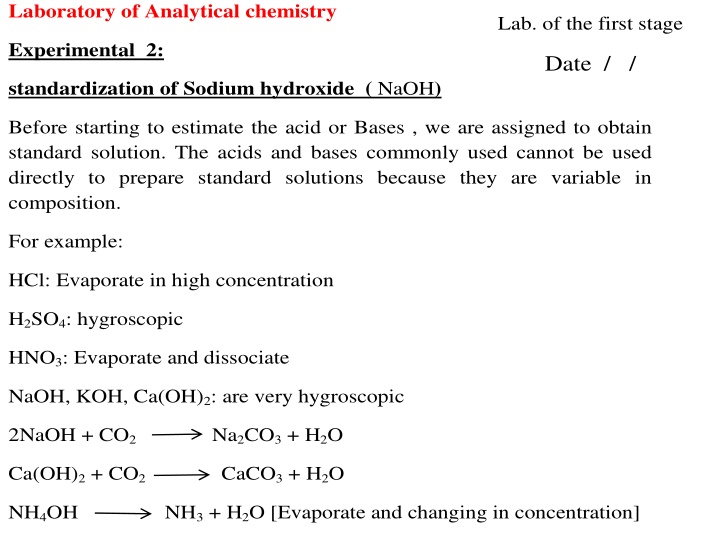
Laboratory of Analytical chemistry Lab. of the first stage Experimental 2: Date / / standardization of Sodium hydroxide ( NaOH) Before starting to estimate the acid or Bases , we are assigned to obtain standard solution. The acids and bases commonly used cannot be used directly to prepare standard solutions because they are variable in composition. For example: HCl: Evaporate in high concentration H2SO4: hygroscopic HNO3: Evaporate and dissociate NaOH, KOH, Ca(OH)2: are very hygroscopic 2NaOH + CO2 Na2CO3 + H2O Ca(OH)2 + CO2 CaCO3 + H2O NH4OH NH3 + H2O [Evaporate and changing in concentration]
Procedure: 1-Weigh 4 gm of NaOH and dissolve it in one liter distilled water. 2- Transfer 10 ml of standard HCl solution to a conical flask. 3- Add 1-2 drops of phenolphthalein (ph.ph) as an indicator. 4- Fill the burette with the prepared NaOH solution. 5- Add NaOH drop by drop into the conical flask until the color of the solution is faint pink. 6- The exact normality of NaOH is obtained from: HCl + NaOH NaCl + H2O N1V1(NaOH)= N2V2(HCl)
An indicator? Is an organic chemical compound weak acid or weak base that exhibits a change in color as a result of concentration changes that occur near the equivalence point. Characteristics of Suitable indicator: 1 - The change in the color of the manual must be clear. 2 - The pH range during which the change in color must be based on the full reaction. Indicators pH Range Change color Acidic medium faint orange red yellow colorless red Basic medium yellow yellow blue red blue 3-4.4 4.4-6.3 6-7.6 8.2-10 6-8 Methyl Orange (M.O) Methyl Red (M.R) Bromothymol blue phenolphthalein (ph.ph) Litmus paper
Titration curve: This is very similar to the previous curve except, of course, that the pH starts off low and increases as you add more sodium hydroxide solution. Again, the pH doesn't change very much until you get close to the equivalence point. Then it surges upwards very steeply.
Homework: Why is a indicator such as phenolphthalein used in neutralizing reactions between a strong Acid and a strong Base ? Write the mechanism of action of the phenolphthalein indicator in neutralizing reactions between a strong acid and a strong base. Explain in detail the neutralization curve between the reaction of a strong acid and a strong base? Lecturer H. N. K. AL-Salman
