Applying the Octet Rule in Lewis Dot Structures
Learn how to apply the octet rule to predict bonding in molecules and draw Lewis Dot Structures for molecules with double and triple bonds. Understand the concept of shared electrons and how atoms combine to achieve a total of 8 valence electrons.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
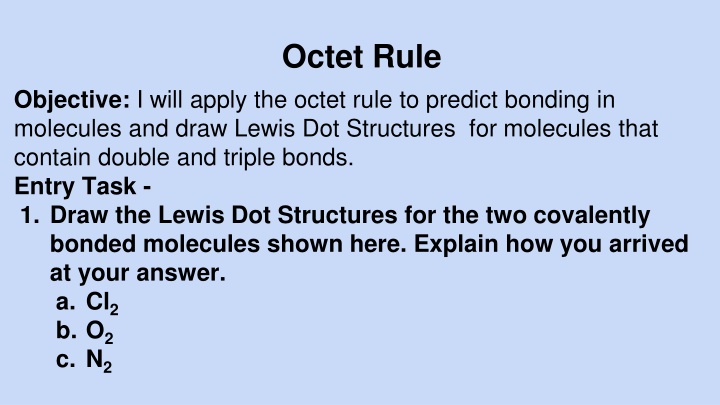
Octet Rule Objective: I will apply the octet rule to predict bonding in molecules and draw Lewis Dot Structures for molecules that contain double and triple bonds. Entry Task - 1. Draw the Lewis Dot Structures for the two covalently bonded molecules shown here. Explain how you arrived at your answer. a. Cl2 b. O2 c. N2
Definitions Octet Rule - Nonmetal atoms combine so that each atom has a total of 8 valence electrons by sharing electrons. (exceptions - H and He are full with 2 e-)
Figuring out structures HONC 1234 - helps you to understand how many bonds an atom can make Lewis Dot Structure - shows that covalent bonds are shared pairs of electrons between two atoms, also shows single electrons that can pair with other single electrons to make covalent bonds Octet Rule - helps you to make sure each atom has the correct number of electrons
Double and Triple Bonds When do you need double/triple bonds to satisfy the octet rule? Do molecules with double/triple bonds satisfy HONC1234? Is it possible to make a triple bonded oxygen molecule? In theory are quadruple bonded carbon molecules possible?
Exit Task 1. Which of these compounds has multiple bonds in it (double or triple bonds)? Explain. C4H10 C4H6 1. Draw one possible structural formula for C4H6






















