Arg-ADNI Study: Data Analysis, Drop-out Rates, and Future Directions
"Explore insights from the Arg-ADNI study on Alzheimer's disease, including data analysis challenges, patient drop-out rates, ongoing 60-month visits, limitations, and future directions for research and grants. Learn from the experiences and lessons of this initiative for improved healthcare strategies. "
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Argentina Arg-ADNI Ezequiel Surace, PhD
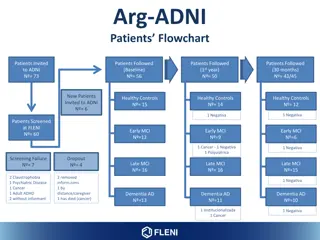
Arg-ADNI Status Baseline Follow-up Follow-up 2012 Total N NPS Ass MRI CSF A -tau PET FDG PET PiB Follow- up 1 year Follow- up 30 month Follow- up 60 month Healthy Controls 15 15 15 11 15 15 13 12 8 Early MCI 12 12 12 7 10 8 10 6 0 Late MCI 16 16 16 14 16 15 16 15 4 Dementia AD 13 13 13 8 12 9 11 10 2 40 53 50 TOTAL 56 56 56 50 43 14 (71%) (95%) (89%)
Arg-ADNI Drop-out rates 2012 2017 2018 Patients Followed (60 months) N = 14 (so far ) Patients Followed (Baseline) N = 56 Patients Followed (30 months) N = 43 Patients Followed (1styear) N = 50 Drop-out rate 10.7% N = 6 Drop-out rate 14% N = 7 Reason for delay of the visit Drop-out: 3 Passed away: 4 Too far from the center: 3 Hospitalized: 4
Arg-ADNI 60-month visit (ongoing) 60-month visit Baseline Follow-up 60 month NPS Ass Alz Costs Survey MRI CSF PET PiB PET-tau AV-1451 (Only for PiB- at baseline) Healthy Controls x x x x 15 8 Early MCI 12 0 Late MCI 16 4 Dementia AD 13 2 TOTAL 56 14
Arg-ADNI Limitations Small cohort generates difficulties in data analysis Baseline Follow-up Initially, our intention was to apply for grants to increase cohort number, but:Available grants contemplate incorporation of preventive interventions. Also, these grants are not sufficient for omics analyses From the participant s standpoint: they manifest less enthusiasm to participate, especially of they are already symptomatic We have not been able to convince other Latin American centers to participate in order to share efforts has funded this study completely
Arg-ADNI Future directions We will complete the 60-month visit (estimated 1 more year) Baseline Follow-up Data analysis will take a further year For now, we do not anticipate to extend the protocol further Based on the limitations, it would be more feasible to apply to preclinical intervention-based grants Arg-ADNI group will participate in FINGER meeting, AA LATAM Face to Face and Alzheimer Prevention Initiative during this meeting
Arg-ADNI What we have learned ADNI served as a model of how other similar initiatives should be implemented and standarized in our institution PiB-PET, Tau-PET, Spanish adaptation of neuropsychological tests, CSF biomarkers (QC program) Administration of data from multiple sources into integrated databases Consolidation of a multi-disciplinary group
IASDA ARGENTINE INITIATIVE FOR THE STUDY OF DOWN SYNDROME AND ALZHEIMER S Established December 2017 3-year longitudinal study CSF biomarkers Yearly clinical / neuropsychological evaluation Confirmatory cytogenetic study DNA / Plasma Structural MRI PiB-PET /FDG-PET
IASDA ARGENTINE INITIATIVE FOR THE STUDY OF DOWN SYNDROME AND ALZHEIMER S Established December 2017 2018-2019 Completed Baseline Participants recruited: 15 Women: 9 Neuropsychological assessment MRI Median age : 41 (21-58) Remaining 10 Recruit 5 more participants