Avalon Pharma - Innovative Health and Beauty Solutions
Avalon Pharma is a dynamic company based in Saudi Arabia, specializing in the development, manufacturing, and marketing of a diverse range of health and beauty brands and generic prescription medicines. With a strong focus on serving the medical, pharmaceutical, and scientific communities, Avalon Pharma is committed to building long-term partnerships and creating innovative solutions. The company has grown significantly since its establishment in 1998 and is known for its expertise in various fields including dermatology, respiratory, internal medicine, and consumer healthcare. Through a dedicated team of over 500 professionals, Avalon Pharma continues to expand its presence in multiple therapeutic categories, both regionally and internationally.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
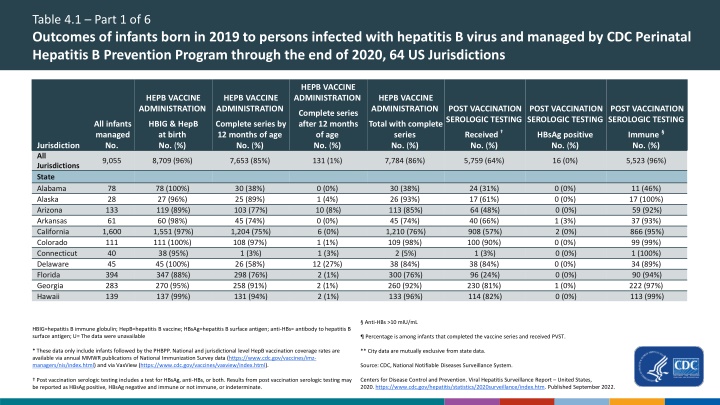
Table 4.1 Part 1 of 6 Outcomes of infants born in 2019 to persons infected with hepatitis B virus and managed by CDC Perinatal Hepatitis B Prevention Program through the end of 2020, 64 US Jurisdictions HEPB VACCINE ADMINISTRATION HEPB VACCINE ADMINISTRATION HEPB VACCINE ADMINISTRATION HEPB VACCINE ADMINISTRATION POST VACCINATION SEROLOGIC TESTING POST VACCINATION SEROLOGIC TESTING POST VACCINATION SEROLOGIC TESTING Complete series after 12 months of age No. (%) All infants managed No. HBIG & HepB at birth No. (%) Complete series by 12 months of age No. (%) Total with complete series No. (%) Received No. (%) Immune No. (%) HBsAg positive No. (%) Jurisdiction All Jurisdictions State Alabama Alaska Arizona Arkansas California Colorado Connecticut Delaware Florida Georgia Hawaii 9,055 8,709 (96%) 7,653 (85%) 131 (1%) 7,784 (86%) 5,759 (64%) 16 (0%) 5,523 (96%) 78 28 133 61 1,600 111 40 45 394 283 139 78 (100%) 27 (96%) 119 (89%) 60 (98%) 1,551 (97%) 111 (100%) 38 (95%) 45 (100%) 347 (88%) 270 (95%) 137 (99%) 30 (38%) 25 (89%) 103 (77%) 45 (74%) 1,204 (75%) 108 (97%) 1 (3%) 26 (58%) 298 (76%) 258 (91%) 131 (94%) 0 (0%) 1 (4%) 10 (8%) 0 (0%) 6 (0%) 1 (1%) 1 (3%) 12 (27%) 2 (1%) 2 (1%) 2 (1%) 30 (38%) 26 (93%) 113 (85%) 45 (74%) 1,210 (76%) 109 (98%) 2 (5%) 38 (84%) 300 (76%) 260 (92%) 133 (96%) 24 (31%) 17 (61%) 64 (48%) 40 (66%) 908 (57%) 100 (90%) 1 (3%) 38 (84%) 96 (24%) 230 (81%) 114 (82%) 0 (0%) 0 (0%) 0 (0%) 1 (3%) 2 (0%) 0 (0%) 0 (0%) 0 (0%) 0 (0%) 1 (0%) 0 (0%) 11 (46%) 17 (100%) 59 (92%) 37 (93%) 866 (95%) 99 (99%) 1 (100%) 34 (89%) 90 (94%) 222 (97%) 113 (99%) Anti-HBs >10 mIU/mL Percentage is among infants that completed the vaccine series and received PVST. ** City data are mutually exclusive from state data. Source: CDC, National Notifiable Diseases Surveillance System. Centers for Disease Control and Prevention. Viral Hepatitis Surveillance Report United States, 2020.https://www.cdc.gov/hepatitis/statistics/2020surveillance/index.htm. Published September 2022. HBIG=hepatitis B immune globulin; HepB=hepatitis B vaccine; HBsAg=hepatitis B surface antigen; anti-HBs= antibody to hepatitis B surface antigen; U= The data were unavailable * These data only include infants followed by the PHBPP. National and jurisdictional level HepB vaccination coverage rates are available via annual MMWR publications of National Immunization Survey data (https://www.cdc.gov/vaccines/imz- managers/nis/index.html) and via VaxView (https://www.cdc.gov/vaccines/vaxview/index.html). Post vaccination serologic testing includes a test for HBsAg, anti-HBs, or both. Results from post vaccination serologic testing may be reported as HBsAg positive, HBsAg negative and immune or not immune, or indeterminate.
Table 4.1 Part 2 of 6 Outcomes of infants born in 2019 to persons infected with hepatitis B virus and managed by CDC Perinatal Hepatitis B Prevention Program through the end of 2020, 64 US Jurisdictions HEPB VACCINE ADMINISTRATION HEPB VACCINE ADMINISTRATION HEPB VACCINE ADMINISTRATION HEPB VACCINE ADMINISTRATION POST VACCINATION SEROLOGIC TESTING POST VACCINATION SEROLOGIC TESTING POST VACCINATION SEROLOGIC TESTING Complete series after 12 months of age No. (%) All infants managed No. HBIG & HepB at birth No. (%) Complete series by 12 months of age No. (%) Total with complete series No. (%) Received No. (%) Immune No. (%) HBsAg positive No. (%) Jurisdiction State Idaho Illinois Indiana Iowa Kansas Kentucky Louisiana Maine Maryland Massachusetts Michigan Minnesota Mississippi Missouri 17 162 117 121 44 60 123 10 253 307 152 398 54 84 16 (94%) 160 (99%) 117 (100%) 119 (98%) 43 (98%) 55 (92%) 108 (88%) 10 (100%) 239 (94%) 303 (99%) 152 (100%) 395 (99%) 51 (94%) 81 (96%) 16 (94%) 144 (89%) 113 (97%) 111 (92%) 39 (89%) 48 (80%) 103 (84%) 10 (100%) 210 (83%) 291 (95%) 140 (92%) 376 (94%) 41 (76%) 72 (86%) 0 (0%) 1 (1%) 0 (0%) 0 (0%) 1 (2%) 2 (3%) 5 (4%) 0 (0%) 7 (3%) 0 (0%) 2 (1%) 5 (1%) 1 (2%) 1 (1%) 16 (94%) 145 (90%) 113 (97%) 111 (92%) 40 (91%) 50 (83%) 108 (88%) 10 (100%) 217 (86%) 291 (95%) 142 (93%) 381 (96%) 42 (78%) 73 (87%) 11 (65%) 99 (61%) 86 (74%) 73 (60%) 27 (61%) 30 (50%) 63 (51%) 7 (70%) 158 (62%) 271 (88%) 121 (80%) 304 (76%) 31 (57%) 48 (57%) 0 (0%) 0 (0%) 1 (1%) 0 (0%) 0 (0%) 0 (0%) 2 (3%) 0 (0%) 0 (0%) 0 (0%) 0 (0%) 0 (0%) 0 (0%) 0 (0%) 11 (100%) 92 (93%) 85 (99%) 68 (93%) 19 (70%) 29 (97%) 57 (90%) 7 (100%) 151 (96%) 265 (98%) 118 (98%) 298 (98%) 29 (94%) 43 (90%) Anti-HBs >10 mIU/mL Percentage is among infants that completed the vaccine series and received PVST. ** City data are mutually exclusive from state data. Source: CDC, National Notifiable Diseases Surveillance System. Centers for Disease Control and Prevention. Viral Hepatitis Surveillance Report United States, 2020.https://www.cdc.gov/hepatitis/statistics/2020surveillance/index.htm. Published September 2022. HBIG=hepatitis B immune globulin; HepB=hepatitis B vaccine; HBsAg=hepatitis B surface antigen; anti-HBs= antibody to hepatitis B surface antigen; U= The data were unavailable * These data only include infants followed by the PHBPP. National and jurisdictional level HepB vaccination coverage rates are available via annual MMWR publications of National Immunization Survey data (https://www.cdc.gov/vaccines/imz- managers/nis/index.html) and via VaxView (https://www.cdc.gov/vaccines/vaxview/index.html). Post vaccination serologic testing includes a test for HBsAg, anti-HBs, or both. Results from post vaccination serologic testing may be reported as HBsAg positive, HBsAg negative and immune or not immune, or indeterminate.
Table 4.1 Part 3 of 6 Outcomes of infants born in 2019 to persons infected with hepatitis B virus and managed by CDC Perinatal Hepatitis B Prevention Program through the end of 2020, 64 US Jurisdictions HEPB VACCINE ADMINISTRATION HEPB VACCINE ADMINISTRATION HEPB VACCINE ADMINISTRATION HEPB VACCINE ADMINISTRATION POST VACCINATION SEROLOGIC TESTING POST VACCINATION SEROLOGIC TESTING POST VACCINATION SEROLOGIC TESTING Complete series after 12 months of age No. (%) All infants managed No. HBIG & HepB at birth No. (%) Complete series by 12 months of age No. (%) Total with complete series No. (%) Received No. (%) Immune No. (%) HBsAg positive No. (%) Jurisdiction State Montana Nebraska Nevada New Hampshire New Jersey New Mexico New York State North Carolina North Dakota Ohio Oklahoma Oregon Pennsylvania 10 62 82 9 (90%) 43 (69%) 82 (100%) 6 (60%) 56 (90%) 81 (99%) 0 (0%) 0 (0%) 0 (0%) 6 (60%) 56 (90%) 81 (99%) 4 (40%) 44 (71%) 59 (72%) 0 (0%) 0 (0%) 0 (0%) 4 (100%) 42 (95%) 58 (98%) 16 16 (100%) 10 (63%) 0 (0%) 10 (63%) 7 (44%) 0 (0%) 7 (100%) 271 11 218 191 36 269 69 50 191 244 (90%) 9 (82%) 205 (94%) 184 (96%) 35 (97%) 264 (98%) 67 (97%) 40 (80%) 189 (99%) 196 (72%) 10 (91%) 201 (92%) 174 (91%) 27 (75%) 223 (83%) 61 (88%) 39 (78%) 171 (90%) 10 (4%) 1 (9%) 1 (0%) 4 (2%) 5 (14%) 2 (1%) 2 (3%) 4 (8%) 4 (2%) 206 (76%) 11 (100%) 202 (93%) 178 (93%) 32 (89%) 225 (84%) 63 (91%) 43 (86%) 175 (92%) 98 (36%) 9 (82%) 166 (76%) 122 (64%) 15 (42%) 113 (42%) 46 (67%) 0 (0%) 113 (59%) 0 (0%) 0 (0%) 0 (0%) 0 (0%) 0 (0%) 2 (2%) 0 (0%) 0 (0%) 0 (0%) 88 (90%) 7 (78%) 163 (98%) 119 (98%) 15 (100%) 102 (90%) 45 (98%) 0 (0%) 112 (99%) Anti-HBs >10 mIU/mL Percentage is among infants that completed the vaccine series and received PVST. ** City data are mutually exclusive from state data. Source: CDC, National Notifiable Diseases Surveillance System. Centers for Disease Control and Prevention. Viral Hepatitis Surveillance Report United States, 2020.https://www.cdc.gov/hepatitis/statistics/2020surveillance/index.htm. Published September 2022. HBIG=hepatitis B immune globulin; HepB=hepatitis B vaccine; HBsAg=hepatitis B surface antigen; anti-HBs= antibody to hepatitis B surface antigen; U= The data were unavailable * These data only include infants followed by the PHBPP. National and jurisdictional level HepB vaccination coverage rates are available via annual MMWR publications of National Immunization Survey data (https://www.cdc.gov/vaccines/imz- managers/nis/index.html) and via VaxView (https://www.cdc.gov/vaccines/vaxview/index.html). Post vaccination serologic testing includes a test for HBsAg, anti-HBs, or both. Results from post vaccination serologic testing may be reported as HBsAg positive, HBsAg negative and immune or not immune, or indeterminate.
Table 4.1 Part 4 of 6 Outcomes of infants born in 2019 to persons infected with hepatitis B virus and managed by CDC Perinatal Hepatitis B Prevention Program through the end of 2020, 64 US Jurisdictions HEPB VACCINE ADMINISTRATION HEPB VACCINE ADMINISTRATION HEPB VACCINE ADMINISTRATION HEPB VACCINE ADMINISTRATION POST VACCINATION SEROLOGIC TESTING POST VACCINATION SEROLOGIC TESTING POST VACCINATION SEROLOGIC TESTING Complete series after 12 months of age No. (%) All infants managed No. HBIG & HepB at birth No. (%) Complete series by 12 months of age No. (%) Total with complete series No. (%) Received No. (%) Immune No. (%) HBsAg positive No. (%) Jurisdiction State Rhode Island South Carolina South Dakota Tennessee Texas Utah Vermont Virginia Washington West Virginia Wisconsin Wyoming 35 64 16 117 539 77 5 243 U 21 142 3 35 (100%) 59 (92%) 16 (100%) 113 (97%) 519 (96%) 76 (99%) 5 (100%) 229 (94%) U (U) 21 (100%) 142 (100%) 3 (100%) 33 (94%) 51 (80%) 16 (100%) 113 (97%) 462 (86%) 71 (92%) 5 (100%) 228 (94%) U (U) 21 (100%) 131 (92%) 2 (67%) 0 (0%) 1 (2%) 0 (0%) 2 (2%) 2 (0%) 1 (1%) 0 (0%) 2 (1%) U (U) 0 (0%) 3 (2%) 1 (33%) 33 (94%) 52 (81%) 16 (100%) 115 (98%) 464 (86%) 72 (94%) 5 (100%) 230 (95%) U (U) 21 (100%) 134 (94%) 3 (100%) 27 (77%) 36 (56%) 10 (63%) 78 (67%) 377 (70%) 43 (56%) 1 (20%) 146 (60%) U (U) 19 (90%) 95 (67%) 1 (33%) 0 (0%) 0 (0%) 0 (0%) 0 (0%) 0 (0%) 0 (0%) 0 (0%) 0 (0%) U (U) 0 (0%) 2 (2%) 0 (0%) 25 (93%) 36 (100%) 10 (100%) 74 (95%) 363 (96%) 43 (100%) 0 (0%) 141 (97%) U (U) 19 (100%) 92 (97%) 1 (100%) Anti-HBs >10 mIU/mL Percentage is among infants that completed the vaccine series and received PVST. ** City data are mutually exclusive from state data. Source: CDC, National Notifiable Diseases Surveillance System. Centers for Disease Control and Prevention. Viral Hepatitis Surveillance Report United States, 2020.https://www.cdc.gov/hepatitis/statistics/2020surveillance/index.htm. Published September 2022. HBIG=hepatitis B immune globulin; HepB=hepatitis B vaccine; HBsAg=hepatitis B surface antigen; anti-HBs= antibody to hepatitis B surface antigen; U= The data were unavailable * These data only include infants followed by the PHBPP. National and jurisdictional level HepB vaccination coverage rates are available via annual MMWR publications of National Immunization Survey data (https://www.cdc.gov/vaccines/imz- managers/nis/index.html) and via VaxView (https://www.cdc.gov/vaccines/vaxview/index.html). Post vaccination serologic testing includes a test for HBsAg, anti-HBs, or both. Results from post vaccination serologic testing may be reported as HBsAg positive, HBsAg negative and immune or not immune, or indeterminate.
Table 4.1 Part 5 of 6 Outcomes of infants born in 2019 to persons infected with hepatitis B virus and managed by CDC Perinatal Hepatitis B Prevention Program through the end of 2020, 64 US Jurisdictions HEPB VACCINE ADMINISTRATION HEPB VACCINE ADMINISTRATION HEPB VACCINE ADMINISTRATION HEPB VACCINE ADMINISTRATION POST VACCINATION SEROLOGIC TESTING POST VACCINATION SEROLOGIC TESTING POST VACCINATION SEROLOGIC TESTING Complete series after 12 months of age No. (%) All infants managed No. HBIG & HepB at birth No. (%) Complete series by 12 months of age No. (%) Total with complete series No. (%) Received No. (%) Immune No. (%) HBsAg positive No. (%) Jurisdiction City** Chicago District of Columbia Houston New York City Philadelphia San Antonio Territory American Samoa Guam N. Mariana Islands Puerto Rico Virgin Islands 102 101 (99%) 78 (76%) 0 (0%) 78 (76%) 70 (69%) 0 (0%) 69 (99%) 27 27 (100%) 25 (93%) 0 (0%) 25 (93%) 22 (81%) 0 (0%) 22 (100%) 119 1,031 128 36 113 (95%) 1,028 (100%) 111 (87%) 36 (100%) 98 (82%) 949 (92%) 103 (80%) 30 (83%) 4 (3%) 5 (0%) 7 (5%) 2 (6%) 102 (86%) 954 (93%) 110 (86%) 32 (89%) 87 (73%) 875 (85%) 84 (66%) 25 (69%) 0 (0%) 0 (0%) 0 (0%) 0 (0%) 85 (98%) 856 (98%) 78 (93%) 25 (100%) 3 3 (100%) 3 (100%) 0 (0%) 3 (100%) 0 (0%) 0 (0%) 0 (0%) 12 12 (100%) 3 (25%) 4 (33%) 7 (58%) 0 (0%) 0 (0%) 0 (0%) 5 5 (100%) 5 (100%) 0 (0%) 5 (100%) 5 (100%) 5 (100%) 0 (0%) 1 1 1 (100%) 1 (100%) 1 (100%) 1 (100%) 0 (0%) 0 (0%) 1 (100%) 1 (100%) 0 (0%) 1 (100%) 0 (0%) 0 (0%) 0 (0%) 1 (100%) Anti-HBs >10 mIU/mL Percentage is among infants that completed the vaccine series and received PVST. ** City data are mutually exclusive from state data. Source: CDC, National Notifiable Diseases Surveillance System. Centers for Disease Control and Prevention. Viral Hepatitis Surveillance Report United States, 2020.https://www.cdc.gov/hepatitis/statistics/2020surveillance/index.htm. Published September 2022. HBIG=hepatitis B immune globulin; HepB=hepatitis B vaccine; HBsAg=hepatitis B surface antigen; anti-HBs= antibody to hepatitis B surface antigen; U= The data were unavailable * These data only include infants followed by the PHBPP. National and jurisdictional level HepB vaccination coverage rates are available via annual MMWR publications of National Immunization Survey data (https://www.cdc.gov/vaccines/imz- managers/nis/index.html) and via VaxView (https://www.cdc.gov/vaccines/vaxview/index.html). Post vaccination serologic testing includes a test for HBsAg, anti-HBs, or both. Results from post vaccination serologic testing may be reported as HBsAg positive, HBsAg negative and immune or not immune, or indeterminate.
Table 4.1 Part 6 of 6 Outcomes of infants born in 2019 to persons infected with hepatitis B virus and managed by CDC Perinatal Hepatitis B Prevention Program through the end of 2020, 64 US Jurisdictions HEPB VACCINE ADMINISTRATION HEPB VACCINE ADMINISTRATION HEPB VACCINE ADMINISTRATION HEPB VACCINE ADMINISTRATION POST VACCINATION SEROLOGIC TESTING POST VACCINATION SEROLOGIC TESTING POST VACCINATION SEROLOGIC TESTING Complete series after 12 months of age No. (%) All infants managed No. HBIG & HepB at birth No. (%) Complete series by 12 months of age No. (%) Total with complete series No. (%) Received No. (%) Immune No. (%) HBsAg positive No. (%) Jurisdiction Freely Associated Island Nations Marshall Islands Micronesia Palau U U (U) U (U) U (U) U (U) U (U) U (U) U (U) 35 3 32 (91%) 2 (67%) 23 (66%) 3 (100%) 2 (6%) 0 (0%) 25 (71%) 3 (100%) 0 (0%) 0 (0%) 0 (0%) 0 (0%) 0 (0%) 0 (0%) Anti-HBs >10 mIU/mL Percentage is among infants that completed the vaccine series and received PVST. ** City data are mutually exclusive from state data. Source: CDC, National Notifiable Diseases Surveillance System. Centers for Disease Control and Prevention. Viral Hepatitis Surveillance Report United States, 2020.https://www.cdc.gov/hepatitis/statistics/2020surveillance/index.htm. Published September 2022. HBIG=hepatitis B immune globulin; HepB=hepatitis B vaccine; HBsAg=hepatitis B surface antigen; anti-HBs= antibody to hepatitis B surface antigen; U= The data were unavailable * These data only include infants followed by the PHBPP. National and jurisdictional level HepB vaccination coverage rates are available via annual MMWR publications of National Immunization Survey data (https://www.cdc.gov/vaccines/imz- managers/nis/index.html) and via VaxView (https://www.cdc.gov/vaccines/vaxview/index.html). Post vaccination serologic testing includes a test for HBsAg, anti-HBs, or both. Results from post vaccination serologic testing may be reported as HBsAg positive, HBsAg negative and immune or not immune, or indeterminate.