AVIATOR Study: Treatment of Genotype 1 HCV Infection
The AVIATOR study focuses on the treatment of chronic hepatitis C virus (HCV) infection in genotype 1 patients using a combination of paritaprevir/ritonavir, ombitasvir, dasabuvir, and ribavirin. The study design involves randomization and open-label treatment with different drug regimens to assess efficacy and safety in treatment-naïve or null responder patients.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
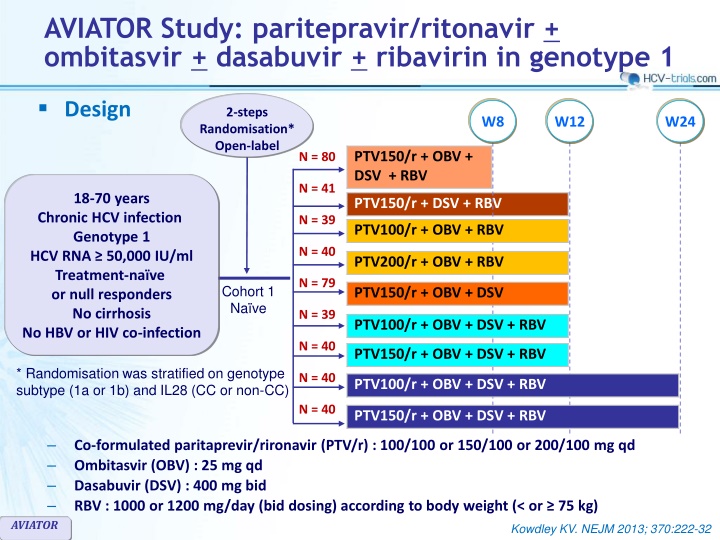
AVIATOR Study: paritepravir/ritonavir + ombitasvir + dasabuvir + ribavirin in genotype 1 Design 2-steps W8 W12 W24 Randomisation* Open-label PTV150/r + OBV + DSV + RBV N = 80 N = 41 18-70 years Chronic HCV infection Genotype 1 HCV RNA 50,000 IU/ml Treatment-na ve or null responders No cirrhosis No HBV or HIV co-infection PTV150/r + DSV + RBV N = 39 PTV100/r + OBV + RBV N = 40 PTV200/r + OBV + RBV N = 79 Cohort 1 Na ve PTV150/r + OBV + DSV N = 39 PTV100/r + OBV + DSV + RBV N = 40 PTV150/r + OBV + DSV + RBV * Randomisation was stratified on genotype subtype (1a or 1b) and IL28 (CC or non-CC) N = 40 PTV100/r + OBV + DSV + RBV N = 40 PTV150/r + OBV + DSV + RBV Co-formulated paritaprevir/rironavir (PTV/r) : 100/100 or 150/100 or 200/100 mg qd Ombitasvir (OBV) : 25 mg qd Dasabuvir (DSV) : 400 mg bid RBV : 1000 or 1200 mg/day (bid dosing) according to body weight (< or 75 kg) AVIATOR Kowdley KV. NEJM 2013; 370:222-32
AVIATOR Study: paritepravir/ritonavir + ombitasvir + dasabuvir + ribavirin in genotype 1 Design Randomisation* 2 : 1 : 1 : 1 : 1 Open-label W12 W24 N = 45 PTV200/r + OBV + RBV 18-70 years Chronic HCV infection Genotype 1 HCV RNA 50,000 IU/ml Treatment-na ve or null responders (NR) No cirrhosis No HBV or HIV co-infection N = 23 PTV100/r + OBV + DSV + RBV N = 22 PTV150/r + OBV + DSV + RBV N = 23 Cohort 2 Prior NR PTV100/r + OBV + DSV + RBV N = 20 PTV150/r + OBV + DSV + RBV * Randomisation was stratified on genotype subtype (1a or 1b) and IL28 (CC or non-CC) Co-formulated paritaprevir/rironavir (PTV/r) : 100/100 or 150/100 or 200/100 mg qd Ombitasvir (OBV) : 25 mg qd ; Dasabuvir (DSV) : 400 mg bid RBV : 1000 or 1200 mg/day (bid dosing) according to body weight (< or 75 kg) Objective Primary analysis : SVR24with two-sided 95% CI, of na ve patients PTV150/r + OBV + DSV + RBV 8 weeks vs 12 weeks, 80% power to detect a 24% difference between groups (mITT analysis) AVIATOR Kowdley KV. NEJM 2013; 370:222-32
AVIATOR Study: paritepravir/ritonavir + ombitasvir + dasabuvir + ribavirin in genotype 1 Baseline characteristics and patient disposition Treatment-na ve N = 438 Null responders N = 133 Mean age, years 50.1 51.1 Female 47% 38% Race : black 14% 14% Genotype 1a 68% 61% IL28B genotype CC 28% 3% HCV RNA log10IU/ml, mean HOMA-IR 3 6.53 6.64 25% 30% 29% 50% Fibrosis score F2 Discontinued treatment, N (%) 24 (5.5%) 5 (3.8%) Adverse event Withdrew consent Lost to follow-up Other 1 0 0 4 0 3 8 13 AVIATOR Kowdley KV. NEJM 2013; 370:222-32
AVIATOR Study: paritepravir/ritonavir + ombitasvir + dasabuvir + ribavirin in genotype 1 SVR24 (HCV RNA < 25 IU/ml) PTV/r + OBV + DSV + RBV 8W PTV/r + OBV + RBV 12W PTV/r + OBV + RBV 12W PTV/r + OBV + DSV 12W PTV/r + OBV + DSV + RBV 12W PTV/r + OBV + DSV + RBV 24W % 96 95 93 91 100 89 89 89 88 83 75 50 25 N 80 41 79 79 79 80 45 45 43 0 Treatment-na ve Null response to prior treatment SVR24in na ve cohort genotype 1a (76-94%) vs 1b (96-100%); odds ratio : 0.087 [p = 0.0008]) AVIATOR Kowdley KV. NEJM 2013; 370:222-32
AVIATOR Study: paritepravir/ritonavir + ombitasvir + dasabuvir + ribavirin in genotype 1 Comparison of SVR24 in Treatment-Na ve Patients, according to treatment Regimens Comparator Group vs Group 5 Difference in rate of SVR24 (95 % CI) Comparison SVR24 p Group 1 vs Group 5 8 W vs 12 W 88% vs 96% 0,08 Group 2 vs Group 5 Contribution of OBV 83% vs 96% 0,06 Group 3 vs Group 5 Contribution of DSV 89% vs 96% 0,09 Group 4 vs Group 5 Contribution of RBV 89% vs 96% 0,09 Group 6 vs Group 5 24 W vs 12 W 91% vs 96% 0,24 -30 -20 -10 0 10 Group 5 better Comparator group better AVIATOR Kowdley KV. NEJM 2013; 370:222-32
AVIATOR Study: paritepravir/ritonavir + ombitasvir + dasabuvir + ribavirin in genotype 1 Resistance emergence No variants at relapse in 7/10 patients in the 8-week groups In the 12-week and 24-week groups, 31/32 samples at breakthrough or relapse showed emergence of resistant variants. Most common : NS3 : position 168 NS5A : positions 28 and 30 NS5B : position 556 AVIATOR Kowdley KV. NEJM 2013; 370:222-32
AVIATOR Study: paritepravir/ritonavir + ombitasvir + dasabuvir + ribavirin in genotype 1 Adverse events Treatment-Na ve Null responder P/r/O/ D/R-8 P/r/D/ R-12 P/r/O/ R-12 P/r/O/ D-12 P/r/O/D /R-12 P/r/O/D/ R-24 P/r/O/ R-12 P/r/O/D /R-12 P/r/O/D /R-24 Treatment group Discontinuation due to AE 1 0 0 0 2 3 1 0 1 Serious AE 0 0 2 2 1 1 0 0 2 Common AE (> 20%) Fatigue Headache Nausea Insomnia Diarrhea Asthenia Cough 36% 35% 15% 12% 10% 9% 15% 32% 32% 17% 20% 24% 2% 12% 28% 29% 20% 11% 10% 10% 14% 20% 19% 14% 8% 16% 6% 3% 28% 27% 24% 20% 13% 4% 10% 38% 36% 25% 25% 14% 15% 15% 27% 33% 13% 18% 16% 22% 16% 27% 29% 20% 13% 18% 9% 7% 21% 33% 19% 16% 19% 9% 21% P/r : co-formulated paritaprevir/rironavir : 100/100 or 150/100 or 200/100 mg qd O : ombitasvir ; D : dasabuvir ; R : ribavirin; 8 : 8 weeks ; 12 : 12 weeks ; 24 : 24 weeks Grade 3-4 laboratory abnormalities Grade 3 elevation of bilirubin, n = 11 (2%) with no concomitant AST/ALT elevation Grade 3 ALT elevation, n = 5 (1%), Grade 3-4 Triglycerides, n = 7 (1%), Anemia : 5% AVIATOR Kowdley KV. NEJM 2013; 370:222-32
AVIATOR Study: paritepravir/ritonavir + ombitasvir + dasabuvir + ribavirin in genotype 1 Summary In this phase IIb study, all-oral regimens of antiviral agents and RBV were effective both in non-cirrhotic patients with HCV genotype 1 infection who had not received therapy previously and in those who had not had a response to prior therapy Rates of SVR24ranged from 83% to 100% Among previously untreated patients, the rate of treatment failure was lower among those receiving the triple combination of paritaprevir/ritonavir + ombitasvir + dasabuvir + RBV for 12 weeks than among those who received the same regimen for 8 weeks and among those who received fewer agents; extending the treatment to 24 weeks offered no further benefit Higher number of relapses in 3D + RBV 8 weeks vs 12 weeks Paritaprevir/ritonavir + ombitasvir + dasabuvir + RBV for 12 weeks was associated with SVR24of 93% in null responders to prior therapy For genotype 1b, all regimens led to SVR24of 94%-100% with only 1/24 relapse in the 8-week group AVIATOR Kowdley KV. NEJM 2013; 370:222-32





