Basics of Elements, Atoms, and Nucleus
Explore the fundamental concepts of elements, atoms, and nucleus. Discover how all matter is composed of atoms, the three basic particles of an atom, and the significance of the nucleus. Learn about the origins of elements in our universe and the essential roles certain elements play in our daily lives. Uncover the fascinating realm of chemistry with this insightful guide.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Elements File:Barium unter Argon Schutzgas Atmosph re.jpg File:Hydrogen discharge tube.jpg File:Copper.jpg File:HEUraniumC.jpg File:Bromine vial in acrylic cube.jpg File:HeTube.jpg Examples of certain chemical elements. From left to right: hydrogen, barium, copper, uranium, bromine, and helium.
What is an element? A element is a pure substance made of one type of atom Elements are divided into metals and non- metals Examples of non-metal elements include carbon, oxygen, hydrogen, and nitrogen Examples of metal elements include aluminum, iron, copper, and gold A gold bar
Atoms All the matter we can see is made of very small particles called atoms. They are not the smallest things we know of, but they are so small we can t see them except with the most powerful microscopes. (A microscope is a tool for looking at very small things.) There are about 5.07 x 1024or 5,070,000,000,000,000,000,000,000 atoms in a milliliter of water.
All atoms are made up of just 3 basic particles: Name: Proton Charge: +1 Name: Neutron Charge: 0 Name: Electron Charge: -1 Charge causes objects to experience a force when near other electrically charged objects. It s like a magnet.
Inside an atom An atom has a nucleus in the middle, and electrons around the nucleus The nucleus is made of protons and neutrons An atom has the same number of protons as electrons so the + and - charges cancel to equal 0 charge for the atom Question: Is the nucleus charge positive or negative? A helium atom
Where did the elements come from? The elements on our planet were mostly made before the Earth was made The simplest elements, hydrogen and helium, were created when the universe was created The other elements were made inside stars which later exploded Most of your body s atoms were made inside stars billions of years ago
What can elements do? Some elements we need to stay alive, like oxygen. Oxygen is in air, and we would die in minutes without it Other elements are very poisonous and even a small amount would kill us, like arsenic
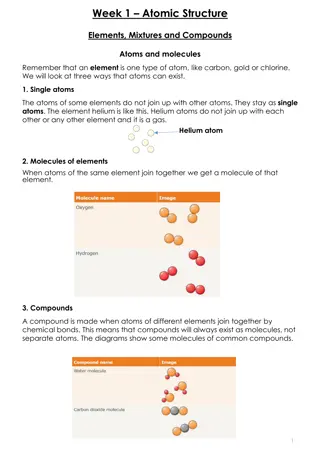
Molecules A molecule is a group of atoms that have joined together into one piece. Molecules have 2 or more atoms in them, and some molecules have thousands or more atoms in them. Here are some common molecules: http://upload.wikimedia.org/wikipedia/commons/thumb/6/6c/Nitrogen-3D-vdW.png/150px-Nitrogen-3D-vdW.png http://upload.wikimedia.org/wikipedia/commons/thumb/1/1c/Water_molecule_3D.svg/121px-Water_molecule_3D.svg.png http://upload.wikimedia.org/wikipedia/commons/thumb/a/af/Carbon-dioxide-3D-vdW.svg/121px-Carbon-dioxide-3D-vdW.svg.png Water: H2O Nitrogen: N2 Carbon Dioxide: CO2
Pure substances A pure substance can be a solid, liquid, or gas. A pure substance has all the same atoms or molecules. An example of a pure substance is pure water. Here are some pure substances: sugar, ice cubes, and baking soda
Mixtures A mixture is matter made up of two or more different types of particles. A mixture can be separated into its different types of particles. An example of a mixture is salt water. You can separate the salt from the water if you evaporate the water. This is a mixture of different candies.
Carbon! Carbon is the element most important to life because it is good at making very large molecules like DNA Pencils use pure carbon (graphite) to write Diamonds are also pure carbon Structure of graphite Structure of DNA
Table salt: Friend or enemy? Some elements are safe sometimes and dangerous other times Table salt is made of sodium and chlorine, but pure sodium will explode if it touches water and chlorine is a poisonous gas used in warfare. + = Chlorine gas was used in battle in World War I A small piece of sodium exploding in water Table salt