Batch and Flow Reactor Design Equations and Sizing Techniques
Explore the design equations and conversion calculations for batch and flow reactors, along with numerical evaluation methods for integrals. Learn how to determine reactor volumes for achieving specific conversions and understand the principles behind reactors in series. Visual aids and formulas provided for better comprehension.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
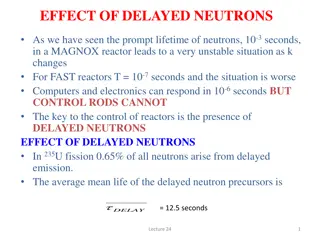
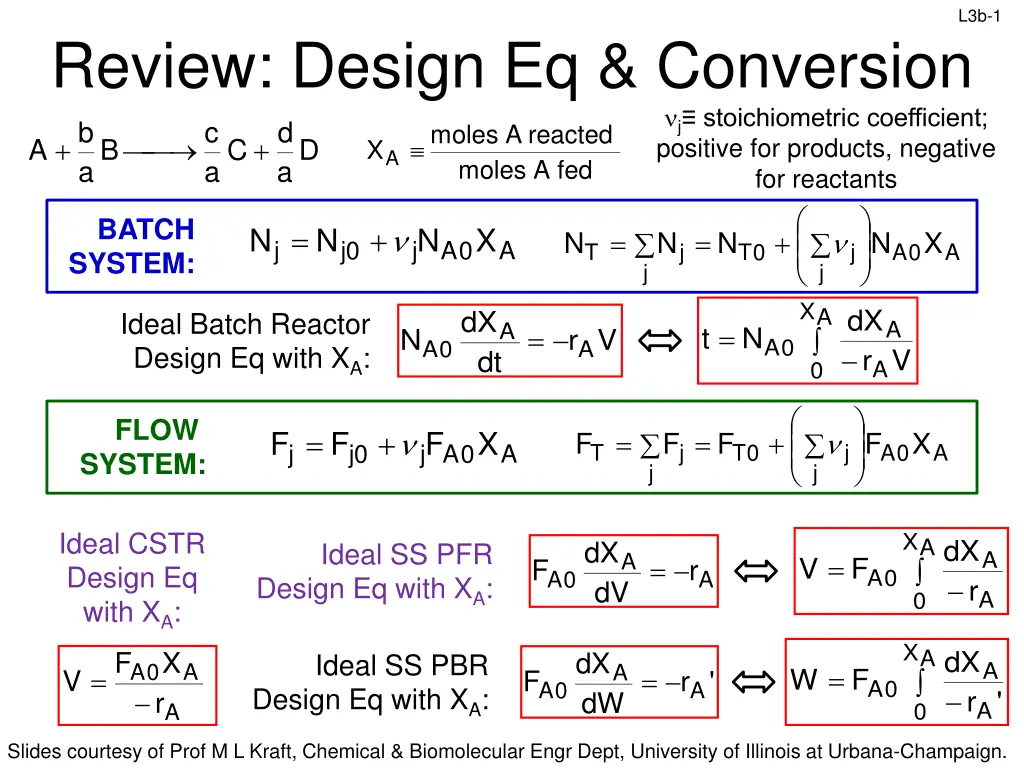
L3b-1 Review: Design Eq & Conversion d C a a moles j stoichiometric coefficient; positive for products, negative for reactants b c moles reacted A + + A B D XA A fed a BATCH SYSTEM: = + X N N N X = = + N N N N X j j 0 j j A 0 A T j T 0 j A 0 A j A dX dX Ideal Batch Reactor Design Eq with XA: A V A = t N = N r V 0 A 0 A 0 A r dt A FLOW SYSTEM: = + F F F X = = + F F F F X j j 0 j j A 0 A T j T 0 j A 0 A j Ideal CSTR Design Eq with XA: F V X A dX dX Ideal SS PFR Design Eq with XA: A A = V F = F r 0 A 0 A 0 A r dV A X A dX X dX Ideal SS PBR Design Eq with XA: A A 0 A A = = W F = F r ' 0 A 0 A 0 A r ' r dW A A Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois at Urbana-Champaign.
L3b-2 Review: Sizing CSTRs We can determine the volume of the CSTR required to achieve a specific conversion if we know how the reaction rate rj depends on the conversion Xj Ideal SS CSTR design eq. Volume is product of FA0/-rA and XA F X F A 0 A A r 0 = = CSTR V CSTR V X A r A A Plot FA0/-rA vs XA (Levenspiel plot) VCSTR is the rectangle with a base of XA,exit and a height of FA0/-rA at XA,exit Area = Volume of CSTR FA0 rA FA0 rA V = X1 X1 X1 X Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois at Urbana-Champaign.
L3b-3 Review: Sizing PFRs & PBRs We can determine the volume (catalyst weight) of a PFR (PBR) required to achieve a specific Xj if we know how the reaction rate rj depends on Xj exit , A X 0 A PFR F V = design eq. X Ideal PFR A exit , F dX A r 0 A dX = PFR V A r A A 0 , 0 A X X A exit exit , F dX Ideal PBR design eq. A r 0 A dX = = W F W PBR A 0 PBR A r A A 0 0 Plot FA0/-rA vs XA (Experimentally determined numerical values) VPFR (WPBR) is the area under the curve FA0/-rA vs XA,exit Area = VPFR or Wcatalyst, PBR Area = Volume of PFR dX r 0 A FA0 rA dX W X FA0 rA F 1 X X1 F 1 A 0 V = = V A 0 dX = 0 r ' A 0 X1 Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois at Urbana-Champaign.
L3b-4 Numerical Evaluation of Integrals (A.4) Simpson s one-third rule (3-point): 2 X f ( ) ( ) ( ) 1 0 0 2 0 1 X X h = Trapezoidal rule (2-point): 1 X h dx x f = h ( ) x ( ) ( ) ( ) + = + + f X f X dx f X f 4 X f X 0 1 2 3 0 X X 2 0 = = + h X X h 1 0 2 Simpson s three-eights rule (4-point): = + = + X X h X X 2 h 1 0 2 0 X 3 3 3 ( ) x ( ) ( ) ( ) ( ) X X = + + + f dx h f X f 3 X f 3 X f X 0 = h 0 1 2 3 8 3 0 Simpson s five-point quadrature : 0 X f 3 X 4 X X 4 h ( ) x ( ) ( ) ( ) ( ) ( ) 0 = h = + + + + f dx f 4 X f 2 X f 4 X f X 1 2 3 4 4 0 Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois at Urbana-Champaign.
L3b-5 Review: Reactors in Series F A0 If is monotonically increasing then: r - A 2 CSTRs 2 PFRs + PFR V PFR V CSTR V i j + CSTR V VCSTR2 PFR V CSTR V VPFR2 VCSTR1 i j VPFR1 PFR CSTR CSTR PFR VCSTR1 + VPFR2 VPFR1 + CCSTR2 VCSTR2 VCSTR1 VPFR2 VPFR1 Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois at Urbana-Champaign.
L3b-6 Chapter 2 Examples Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois at Urbana-Champaign.
L3b-7 Calculate the reactor volumes for each configuration shown below for the reaction data in the table when the molar flow rate is 52 mol/min. -rA is in terms of mol/dm3 s X1=0.3 FA0, X0 X1=0.3 FA0, X0 X2=0.8 X2=0.8 Config 2 Config 1 XA -rA 0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.85 0.0053 0.0052 0.0050 0.0045 0.0040 0.0033 0.0025 0.0018 0.00125 0.001 X F A exit , ( ) F A r 0 Use numerical methods to solve = CSTR V X X A r 0 dX = PFR V A out , A in , A n n A A n X A in , XA,out and XA,in respectively, are the conversion at the outlet and inlet of reactor n 1. Calculate FA0/-rA for each conversion value in the table FA0/-rA mol 52 F0 A = Convert to seconds min 1 60 mol m min mol s = . F = 52 0 8 67 A 0 in s Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois at Urbana-Champaign.
L3b-8 Calculate the reactor volumes for each configuration shown below for the reaction data in the table when the molar flow rate is 52 mol/min. -rA is in terms of mol/dm3 s X1=0.3 FA0, X0 X1=0.3 FA0, X0 X2=0.8 X2=0.8 Config 2 Config 1 XA -rA 0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.85 0.0053 0.0052 0.0050 0.0045 0.0040 0.0033 0.0025 0.0018 0.00125 0.001 164 exit , A X 0 A dX r FA0/-rA F ( ) F A r 0 Use numerical methods to solve = CSTR V X X = PFR V A out , A in , A n n A A n X A in , XA,out and XA,in respectively, are the conversion at the outlet and inlet of reactor n 1. Calculate FA0/-rA for each conversion value in the table mol 52 F0 Convert to seconds mol s 0.867 A = F min 1 60 3 A 0 164 164 d m = = mol m r mol m min mol s = 0.0053 A ( 0) . F = 52 0 8 67 A 0 3 in s d s Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois at Urbana-Champaign.
L3b-9 Calculate the reactor volumes for each configuration shown below for the reaction data in the table when the molar flow rate is 52 mol/min. -rA is in terms of mol/dm3 s X1=0.3 FA0, X0 X1=0.3 FA0, X0 X2=0.8 X2=0.8 Config 2 Config 1 XA -rA 0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.85 0.0053 0.0052 0.0050 0.0045 0.0040 0.0033 0.0025 0.0018 0.00125 0.001 FA0/-rA X F A exit , ( ) F A r 0 Use numerical methods to solve = CSTR V X X A r 0 dX = PFR V A out , A in , A n n A A n X A in , XA,out and XA,in respectively, are the conversion at the outlet and inlet of reactor n 1. Calculate FA0/-rA for each conversion value in the table mol 52 F0 Convert to seconds mol s 0.867 A = For each rA that corresponds to a XA value, use FA0 to calculate F min 1 60 3 A 0 164 164 d m = = mol m r mol m min mol s = 0.0053 A ( 0) FA0/-rA & fill in the table . F = 52 0 8 67 A 0 3 in s d s Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois at Urbana-Champaign.
L3b-10 Calculate the reactor volumes for each configuration shown below for the reaction data in the table when the molar flow rate is 52 mol/min. -rA is in terms of mol/dm3 s X1=0.3 FA0, X0 X1=0.3 FA0, X0 X2=0.8 X2=0.8 Config 2 Config 1 XA -rA 0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.85 0.0053 0.0052 0.0050 0.0045 0.0040 0.0033 0.0025 0.0018 0.00125 0.001 164 167 173 193 217 exit , A X 0 A dX r FA0/-rA 263 347 482 F 694 867 ( ) F A r 0 Use numerical methods to solve = CSTR V X X = PFR V A out , A in , A n n A A n X A in , XA,out and XA,in respectively, are the conversion at the outlet and inlet of reactor n 1. Calculate FA0/-rA for each conversion value in the table mol 52 F0 mol s mol dm A = 0.867 Convert to seconds F min 1 60 3 A0 867 dm = = mol m min mol s = r . F A ( 0.85) = 0.001 52 0 8 67 A 0 in s 3 s Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois at Urbana-Champaign.
L3b-11 0.85 XA -rA 0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.0053 0.0052 0.0050 0.0045 0.0040 0.0033 0.0025 0.0018 0.00125 0.001 164 167 173 193 217 X1=0.3 X2=0.8 FA0/-rA 263 347 482 694 867 FA0, X0 Config 1 Reactor 1, PFR from XA0=0 to XA=0.3: XA,exit PFRn XA,in PFR1 CSTR2 F Use numerical methods to solve A r 0 V dX = A A 4-pt rule: 0.3 F F F F F 3 0.3 8 0 A0 r A0 r A r 0 A0 r A0 r V dX 3 3 = = + + + 0 PFR1 A 3 A A AX A A X 0 0 . 1 X 0.2 = = = A A A X 0.3 = A F 3 8 ( ) ( ) ( ) 0.3 A0 0 3 = = + + + = 16 PFR V dX 0.1 4 3 167 3 173 1 93 5 1.6 dm A r 1 A F ( ) A0 r ( ) 3 = CSTR V X X = = CSTR V 694 0.8 0.3 347 dm A,o ut A i ,n 2 2 A X A,out Total volume for configuration 1: 51.6 dm3 + 347 dm3 = 398.6 dm3 = 399 dm3 Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois at Urbana-Champaign.
L3b-12 0.85 XA -rA 0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.0053 0.0052 0.0050 0.0045 0.0040 0.0033 0.0025 0.0018 0.00125 0.001 164 167 173 193 217 FA0/-rA 263 347 482 694 867 Must evaluate as many pts as possible when the curve isn t flat X1=0.3 FA0, X0 X2=0.8 Config 2 Reactor 1, CSTR from XA0=0 to XA=0.3: F V r ( = CSTR 0. 193 V CSTR1 PFR2 ( ) A 0 X X = CSTR A,out A 1 0 A . 0 3 0.8 F A0 r ) 3 = 0.3 0. 5 PFR2 V dX = 3 0 58 dm A A 0 . 8 F F Need to evaluate at 6 pts, but since there is no 6-pt rule, break it up A0 r A0 r = + V dX dX . PF R2 A A A A 0.3 0 5 . . . . 3 0 5 0 3 0 8 0 5 8 3 3 ( ) 4 ( ) 3 ( ) 3 173 dm = V = + + + + + + 193 217 263 263 34 7 482 694 ( ) 3 2 PFR 3 point rule 4 point rule Total volume for configuration 2: 58 dm3 + 173 dm3 = 231 dm3 Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois at Urbana-Champaign.
L3b-13 For a given CA0, the space time needed to achieve 80% conversion in a CSTR is 5 h. Determine (if possible) the CSTR volume required to process 2 ft3/min and achieve 80% conversion for the same reaction using the same CA0. What is the space velocity (SV) for this system? V 0 F r h space time holding time mean residence time = = 5 V C C CSTR 0 A r 0 A A X 0 0 0 = V X V X = = A CSTR A CSTR A r A A A V C CSTR 0 A r 0 X = = =5 h XA=0.8 0=2 ft3/min A A 3 ft min V 2 60 ( ) = = V h V = 3 5 V ft = 600 0 min h 0 = Space velocity: 1 1 1 -1 0 SV . h 0 2 SV = = = = h V 5 Notice that we did not need to solve the CSTR design equation to solve this problem. Also, this answer does not depend on the type of flow reactor used. Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois at Urbana-Champaign.
L3b-14 A product is produced by a nonisothermal, nonelementary, multiple-reaction mechanism. Assume the volumetric flow rate is constant & the same in both reactors. Data for this reaction is shown in the graph below. Use this graph to determine which of the 2 configurations that follow give the smaller total reactor volume. X1=0.3 FA0, X0 X1=0.3 FA0, X0 X2=0.7 X2=0.7 Config 1 Config 2 F C ( ) A r A r 0 0 V X V X X = = CSTR A CSTR A,out A,in 0 A A Shown on graph XA,exit XA,exit C F A r 0 A r V dX 0 = V dX = PFR A 0 PFRn A X A X A A,in A,in Since 0 is the same in both reactors, we can use this graph to compare the 2 configurations PFR- volume is 0 multiplied by the area under the curve between XA,in & XA,out CSTR- volume is 0 multiplied by the product of CA0/-rA,outlet times (XA,out - XA,in) Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois at Urbana-Champaign.
L3b-15 A product is produced by a nonisothermal, nonelementary, multiple-reaction mechanism. Assume the volumetric flow rate is constant & the same in both reactors. Data for this reaction is shown in the graph below. Use this graph to determine which of the 2 configurations that follow give the smaller total reactor volume. X1=0.3 FA0, X0 X1=0.3 FA0, X0 X2=0.7 X2=0.7 Config 1 Config 2 Config 2 XA = 0.7 Config 1 XA = 0.7 XA = 0.3 XA = 0.3 PFR- V is 0 multiplied by the area under the curve between XA,in & XA,out CSTR- V is 0 multiplied by the product of CA0/-rA,outlet times (XA,out - XA,in) Less shaded area Config 2 (PFRXA,out=0.3 first, and CSTRXA,out=0.7 second) has the smaller VTotal Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois at Urbana-Champaign.