Benzene and Its Derivatives: Orientation and Reactivity Explained
Explore the effects of substituents on monosubstituted benzene, learn about the reactivity and orientation in benzene derivatives, and understand the formation of disubstituted benzene compounds. Delve into the structure and uses of compounds like DDT and BHC in this comprehensive study.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Benzene and its derivatives Dr. Zamir G Khan Assistant Professor H R Patel Institute of Pharmaceutical Education and Research Shirpur, Dist. Dhule, (M.S) 425405
Learning Outcomes After completion of this topic students shall be able to Explain the effects of substituents on Orientation of monosubstituted benzene Explain the effects of substituents on Reactivity of monosubstituted benzene Discuss the structure and uses of DDT (Dichlorodiphenyltrichloroethane), Saccharin Discuss the structure and uses of BHC (Benzene hexachloride), Chloramine 19-04-2025 2
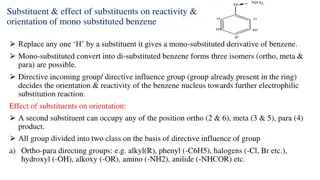
Orientation and Reactivity in monosubstituted benzene E Electrophilic substitution G G G G E Electrophilic substitution + + E Ortho (1,2) E Meta (1,3) Para (1,4) 19-04-2025 3
Orientation and Reactivity in monosubstituted benzene 1. Electrophilic substitution reaction results in formation of monosubstituted benzene 2. Electrophilic substitution reaction of monosubstituted benzene results in formation of three possible disubstituted benzene 3. With respect to the group already present, reaction results in formation of three possible disubstituted benzene (o, m, p) 19-04-2025 4
NO2 Conc. HNO3+H2SO4 60oC, 1 hr Monosubstituted 1 Nitrobenzene CH3 CH3 CH3 CH3 NO2 Conc. HNO3+H2SO4 60oC, 10 min. Ortho Para Directing + + 2 NO2 o-nitrotoluene (57%) m-nitrotoluene (3%) NO2 p-nitrotoluene (40%) NO2 NO2 NO2 NO2 NO2 3 Conc. HNO3+H2SO4 90oC-100oC, reflux Meta Directing + + NO2 o-dinitrobenzene (6.4%) m-dinitrobenzene (93.3%) NO2 p-dinitrobenzene (0.3%) 19-04-2025 5
Orientation and Reactivity in monosubstituted benzene Mentimeter Question 19-04-2025 6
Orientation and Reactivity in monosubstituted benzene Mentimeter Question 19-04-2025 7
Orientation and Reactivity in monosubstituted benzene The group already attached to benzene ring governs two important factors 1. Reactivity 1. Ring activator 2. Ring deactivator 2. Orientation 1. Ortho para directing 2. Meta directing 19-04-2025 8
Orientation and Reactivity in monosubstituted benzene 19-04-2025 9
Orientation and Reactivity in monosubstituted benzene Nature of groups: 1. Electron releasing group (+I) (+R): -OH, -NH2, -NHR, -NR2, -CH3, -OCH3, -Cl, -Br, -I, -F 2. Electron withdrawing group (-I) (-R): -NO2, -CN, -C=O, -CHO, -COOH, -COOR,-SO3H 19-04-2025 10
Effect of group on Reactivity in monosubstituted benzene 19-04-2025 11
Effect of group on Reactivity in monosubstituted benzene 19-04-2025 12
Effect of group on Reactivity in monosubstituted benzene 19-04-2025 13
Effect of group on Reactivity in monosubstituted benzene 19-04-2025 14
Example: Chlorination of methylbenzene CH3 CH3 Cl CH3 FeCl3 + Cl2 + Cl o-Chlorotoluene p-Chlorotoluene Ring activator CH3 Ortho Para Director 19-04-2025 15
Example: Nitration of Nitrobenzene NO2 NO2 NO2 NO2 NO2 Conc. HNO3+H2SO4 + + NO2 90oC-100oC, reflux NO2 o-dinitrobenzene (6.4%) p-dinitrobenzene (0.3%) m-dinitrobenzene (93.3%) Ring deactivator NO2 Meta Director 19-04-2025 16
Halogens are ortho para director but ring deactivator 19-04-2025 17
Thank you Mr. Z. G. Khan, Assist. Professor, H R Patel Institute of Pharmaceutical Education and Research Shirpur, Dist Dhule (M.S) 425 405 Email: khanzamir.5588@gmail.com Webpage: https://khanzamir5588.wixsite.com/zamir YouTube Channel: https://www.youtube.com/channel/UCsQae74- x4t5iYK02iPRTxA?view_as=subscriber Whatsapp: +91 9890 044 661 19-04-2025 18