Blunt-End Ligation: Advantages, Disadvantages & Modifications
Blunt-end ligation in molecular biology, while inefficient, offers universality in ligations. Learn about advantages, disadvantages, and modifications to enhance ligation frequency.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
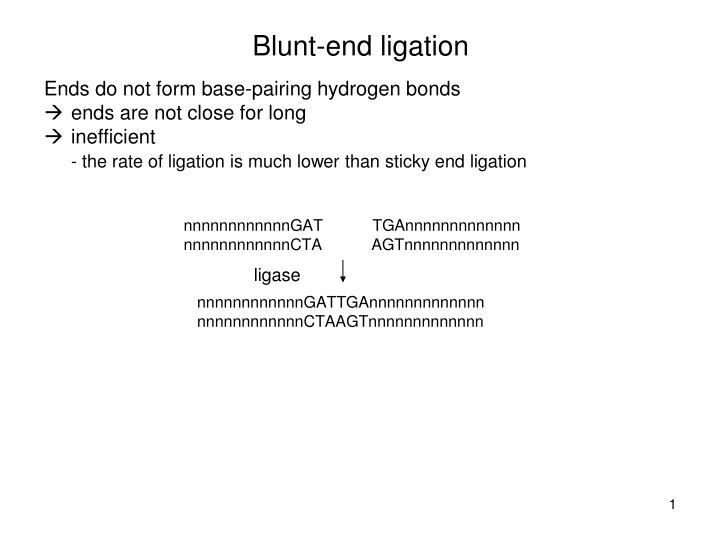
Blunt-end ligation Ends do not form base-pairing hydrogen bonds ends are not close for long inefficient - the rate of ligation is much lower than sticky end ligation nnnnnnnnnnnnGAT nnnnnnnnnnnnCTA TGAnnnnnnnnnnnnn AGTnnnnnnnnnnnnn ligase nnnnnnnnnnnnGATTGAnnnnnnnnnnnnn nnnnnnnnnnnnCTAAGTnnnnnnnnnnnnn 1
Why do blunt-end ligations ? Disadvantage: inefficient Advantage: Universal - many ligations can be carried out with the same set-up Blunt-end ligation are commonly used, When sticky ends of insert and vector can not be matched The fragment are then polished enzymatically to generate blunt ends (ex. by using Klenow fragment with DNA polymerase and exonuclease activities), followed by blunt-end ligation To clone PCR products from amplification with polymerases that generate blunt ends (as we have done in the lab before) 2
Simple blunt-end ligation of PCR product (amplicon) into plasmid vector MCS is cut (linearized) with enzyme generating blunt ends PCR amplify gene of interest using Taq polymerase and standard primers plasmid EcoRV endonuclease ligase ATP Ligation of amplicon into plasmid 3
For several reasons, this approach results in low frequency ligation of amplicon into plasmid Taq polymerase lacks proof-reading activity and frequently puts an extra A at the 3 end amplicons are not blunt-ended few/no white colonies EcoRV plasmid endonuclease Standard primers have 5 OH group only one strand can be ligated ligase ATP Ligation of amplicon into plasmid ligase ATP -- 3 OH x HO 5 ----5 P- HO 3 plasmid ends are physically linked plasmids frequently self ligate many blue colonies few/no white colonies concentration of plasmid and especially amplicon is too low for sufficient rate of ligation to occur few/no white colonies 4
Modifications used to increase frequency of amplicon ligation into plasmid We use DNA polymerase with proof-reading activity amplicons are blunt-ended white colonies EcoRV plasmid endonuclease We use primers that have 5 P group both strands can be ligated ligase ATP Ligation of amplicon into plasmid ligase/ATP -- 3 OH - P 5 ---5 P- HO 3 EcoRV ligation is done in the presence of EcoRV self-ligated plasmid will be re-cut few blue colonies white colonies We use high concentration of plasmid and especially amplicon (5:1 to 10:1) higher rate of ligation white colonies 5 We also use highly transformation-competent E. coli cells
when we have colonies we want to check if they have the insert- we make a plasmid prep and cut with REs: Confirm insertion of gene in a plasmid DNA After Digestion Ladder Cut Plasmid: 5327 bp Plasmid Total Plasmid: 6487 bp Gene of Interest: 1160 bp Gene: 1160 bp 6