Bohr Models for Element Reactivity
Explore how Bohr models are used to predict reactivity in elements by analyzing valence electrons. Learn the rules for filling energy levels and practice drawing Bohr models for different elements like Carbon, Sulfur, and Lithium. Enhance your understanding of protons, neutrons, and electrons in atoms through guided practice exercises.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
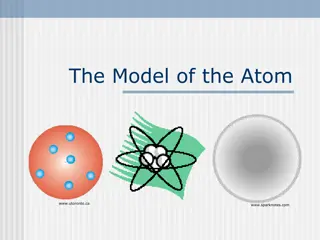
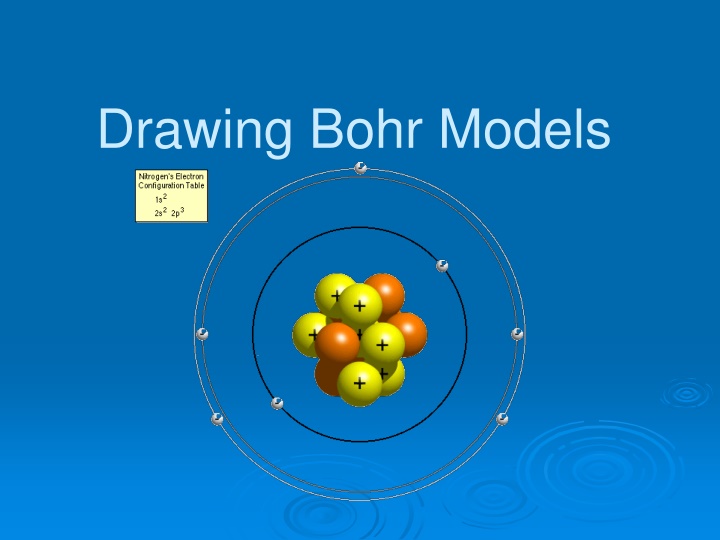
Bohr Models 1. Bohr models are used to predict reactivity in elements. 2. Reactivity refers to how likely an element is to form a compound with another element. 3. When looking at Bohr models, we look at its valence electrons (the electrons on the last energy level) to determine reactivity.
Drawing Bohr Models Draw the nucleus. Write the number of neutrons and the number of protons in the nucleus. Draw the first energy level. Draw the electrons in the energy levels according to the rules below. Make sure you draw the electrons in pairs. Keep track of how many electrons are put in each level and the number of electrons left to use. 1. 2. 3. 4. 5.
Rules for Energy Levels 1. Level 1 (closest to the nucleus) can hold a maximum of 2e. 2. Level 2 can hold a max of 8e. 3. Level 3 can hold a max of 18e. 4. Level 4 can hold a max of 32e. You must fill one level before going on to draw the next level!
Guided Practice In order to draw Bohr models of these elements, you must first determine the number of protons, neutrons, and electrons. Once you have found this information, follow the directions to draw your model. 6 6 6 6 Protons: _____ How many energy shells will this have? ____ How many valence (outer) electrons does this element have? ____ Bohr Model: Neutrons: _____ Electrons: ______ 2 C 4 Carbon 12.011
Guided Practice 16 16 16 16 Protons: _____ How many energy shells will this have? ____ How many valence (outer) electrons does this element have? ____ Bohr Model: Neutrons: _____ Electrons: ______ 3 S 6 Sulfur 32.066
Guided Practice 3 Neutrons: _____ Electrons: ______ 4 3 3 Protons: _____ How many energy shells will this have? ____ How many valence (outer) electrons does this element have? ____ Bohr Model: 2 Li 1 Lithium 6.941
Guided Practice 10 10 10 10 Protons: _____ How many energy shells will this have? ____ How many valence (outer) electrons does this element have? ____ Bohr Model: Neutrons: _____ Electrons: ______ 2 Ne 8 Neon 20.180
Guided Practice 11 12 11 11 Protons: _____ How many energy shells will this have? ____ How many valence (outer) electrons does this element have? ____ Bohr Model: Neutrons: _____ Electrons: ______ 3 Na 1 Sodium 22.990