Bond Dissociation Energy (BDE) and Born-Haber Cycles
Explore the concepts of Bond Dissociation Energy (BDE) and Born-Haber Cycles in chemistry. Learn about the energy required to break chemical bonds, average bond values, and calculation of enthalpy in reactions using BDE. Dive into lattice energy and its significance in ionic bonds. Master these essential topics with examples and detailed explanations.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
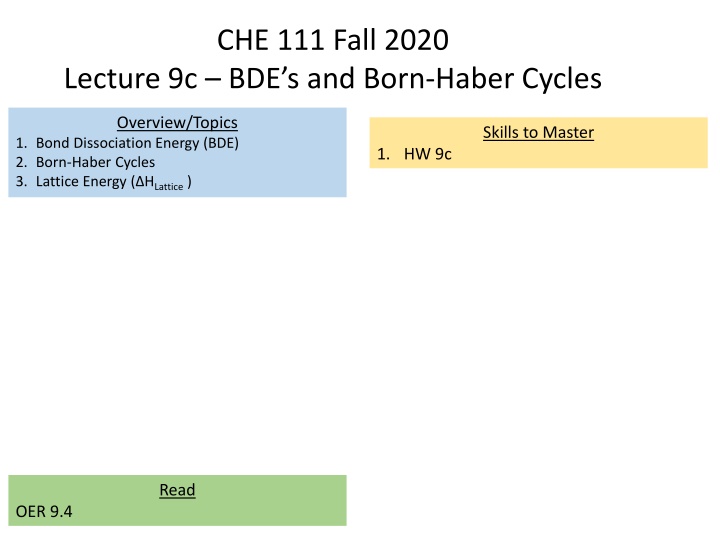
CHE 111 Fall 2020 Lecture 9c BDE s and Born-Haber Cycles Overview/Topics Skills to Master 1. Bond Dissociation Energy (BDE) 2. Born-Haber Cycles 3. Lattice Energy ( HLattice ) 1. HW 9c Read OER 9.4
Review Ch. 5a VBT/MO Bond Dissociation Energies (BDE) o Amount of energy required to break a chemical bond o Average values of many different bonds o Useful because 30 million compounds exist, only a few have experimentally determined values o Tabulate in Table 9.3 p 500 OER Book Cl2(g) _2_ Cl (g) HBDE = 243 kJ/mol
Bond Dissociation Energies (BDE) o # bonds (e- shared) BDE o BDE Down a Column Tabulate in Table 9.3 p 500 OER Book
BDEs and VBT HBDE = 436 kJ/mol H-H H-F HBDE = 569 kJ/mol F-F HBDE = 160 kJ/mol
BDEs and VBT sp hybridization sp2 hybridization sp3 hybridization
Hrxn and BDE Hrxn = BDE - BDE Bonds Broken Bonds Formed Example _1_ N2 (g) + _3_ H2(g) _2_ NH3 (g) Hrxn = Break N=N + Break 3 (H-H) Make 6 N-H Hrxn = 945 kJ + 3 (436) kJ 6 (390) kJ Hrxn =-87 kJ/mol Tabulated Value -92.6 kJ/mol
Example Using BDE s calculate Enthalpy of Reaction for the combustion of butane _ C4H10 (g) + __ O2(g) __ CO2 (g) + __ H2O (g) Tabulated Value -2877 kJ/mol
Lattice Energy (Hlattice) o Energy change when an Ionic chemical bond is broken o Difficult to measure and calculate o DP stability of bond/molecule (more negative more stable) Endothermic (+) Endothermic (+) as defined, some books use the opposite definition CA (s) C+ (g) + A- (g) Exothermic (-) Coulomb's Law C = constant (depends on crystal structure) Z+ = charge cation Z- = charge anion R = bond length (sum of ion radii) Hlattice=C(Z+)(Z ) R DP to charge on ions IP to size of ions
Trends in Lattice Energy Hlattice=C(Z+)(Z ) R Lattice Energy is IP to Size Lattice Energy is DP to Charge -834 kJ/mol E n e r g y S i z e -788 kJ/mol -701 kJ/mol -3414 kJ/mol -910 kJ/mol -657 kJ/mol
Example Which will have a greater Lattice Energy? Explain. (a) MgF2 vs MgI2 (b) NaCl vs MgCl2
Born-Haber Cycles o Hard to measure lattice energies experimentally o Easier to calculate o Similar to Hess s Law o Can solve for any value, though normally its Lattice Energy or Enthalpy of Reaction Hrxn = Hsub + BDE + EIE + EEA + Hlattice Need to know 6 values (Tables in OER Book) Energy Symbol Table Enthalpy of Sublimation (metal) Hsub BDE *Given in problem BDE (nonmetal) Table 9.3 Figure 3.34 Table 3.3 Ionization Energy IE or EIE Electron Affinity EA or EEA Hlattice Hf Figure 3.35 Lattice Energy Not in Book Enthalpy of Formation (rxn) Appendix G *All Values Given for HW and Exam
Ionization Energy * Additional values not from book Use values in book first! Lattice Energy *Not from book, use with care!
Tabulate in Table 9.3 p 500 OER Book Bond Dissociation Energy (BDE)
Example Calculate the Lattice Energy for NaCl using a Born-Haber Cycle. Na (s) + Cl2(g) NaCl (s) Na+1 (g) + Cl-1 (g) NaCl (s) Given: Hrxn = Hsub + BDE + EIE + EEA + Hlattice Hsub (Na) = 107.3 kJ/mol
Step Reaction Process Value (kJ/mol) 1 Na (s) Na (g) Sublimation 107.3 2 Cl2(g) Cl (g) Na (g) Na+1 + e- BDE (243) 3 Ionization Energy 495.8 Cl (g) + e- Cl-1 (g) 4 Electron Affinity -348.6 Na+1 (g) + Cl-1 (g) NaCl (s) 5 Lattice Energy -787 Enthalpy of Reaction 6 Na (s) + Cl2 (g) NaCl (s) -411
Example Calculate the Enthalpy of Reaction for MgCl2 using a Born-Haber Cycle. Mg (s) + Cl2(g) MgCl2 (s) Hrxn = Hsub + BDE + EIE + EEA + Hlattice *-641.8 = google *-642.1 = this problem
Solution: Step Reaction Process Value (kJ/mol) 1 Mg (s) Mg (g) Sublimation 147.1 2 Cl2(g) 2 Cl (g) Mg (g) Mg+1 (g) + e- BDE 243 3a IIE1 IIE2 Electron Affinity 738 Mg+1 (g) Mg+2 (g) + e- 3b 1451 2 Cl (g) + 2e- 2 Cl-1 (g) 4 2(-348.6) Mg+2 (g) + 2 Cl-1 (g) MgCl2 (s) Lattice Energy Mg (s) + Cl2 (g) MgCl2 (s) 5 -2524 Enthalpy of Reaction 6 -642.1 *-641.8 = google *-642.1 = this problem
Compare the two problems Step Reaction Process Value (kJ/mol) 1 Na (s) Na (g) Sublimation 107.3 2 Cl2(g) Cl (g) Na (g) Na+1 + e- BDE (243) 3 Ionization Energy 495.8 Cl (g) + e- Cl-1 (g) 4 Electron Affinity -348.6 Na+1 (g) + Cl-1 (g) NaCl (s) 5 Lattice Energy -787 Enthalpy of Reaction 6 Na (s) + Cl2 (g) NaCl (s) -411 Step Reaction Process Value (kJ/mol) 1 Mg (s) Mg (g) Sublimation 147.1 2 Cl2(g) 2 Cl (g) Mg (g) Mg+1 (g) + e- BDE 243 3a IIE1 IIE2 Electron Affinity 738 Mg+1 (g) Mg+2 (g) + e- 3b 1451 2 Cl (g) + 2e- 2 Cl-1 (g) 4 2(-348.6) Mg+2 (g) + 2 Cl-1 (g) MgCl2 (s) Na (s) + Cl2 (g) NaCl (s) 5 Lattice Energy -2524 Enthalpy of Reaction 6 -642.1
Compare the two problems 1. Step 1 Value always given (not in book) 2. Step 2 BDE (depends on how many atoms you need) multiply by or 1 3. Step 3 - Ionization Energy (# steps depends on number charge of cation) 4. Step 4 Electron Affinity (depends on how many ions you make) multiply by 1 or 2 5. Step 5 Given or Solve for it 6. Step 6 Appendix G or Given or Solve for it
Chapter 10 Enthalpies of Physical Change H2O (l) H2O (g) HVap = 44 kJ/mol H2O (s) H2O (l) HFus = 6.01 kJ/mol 44.01 Enthalpies of Solution Chapter 11