Building Binary Compounds: Understanding Element Combinations
world of binary compounds formed by non-metals, metals, and transition metals. Learn about naming conventions, charges, and formulas. Dive into practice exercises for creating and naming these compounds. Discover the formulas and names of compounds like magnesium oxide and lithium arsenide. Delve into the chemistry behind creating binary compounds from elements like calcium and nitrogen or potassium and phosphorus.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
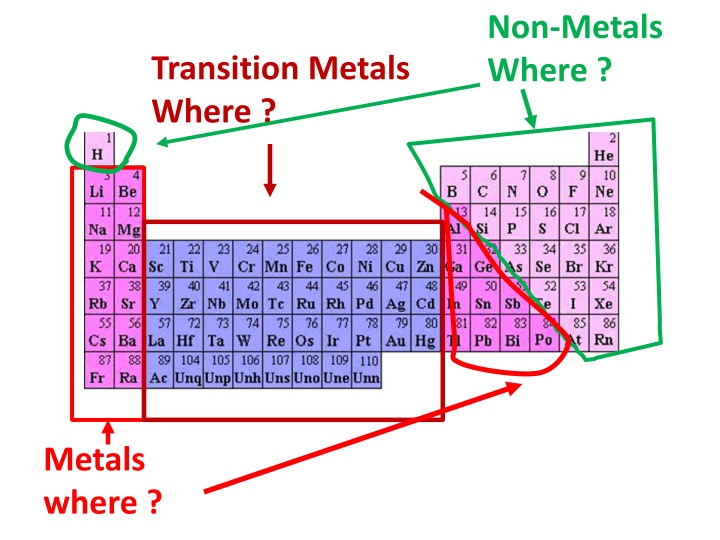
Non-Metals Where ? Transition Metals Where ? Metals where ?
Building Ionic Binaries: Know- Thy Charges (reviewed) 0 -4,+4 +1 +2 -5,+3 -1 -3 -2 +1,2,3,4, (depends)
Binary compound naming 1. Non-Metal + Non-Metal 2. Metal + Non-Metal 3. Transition Metal + Non-Metal = NM+NM = M + NM = TM + NM
Building and Naming M + NM +1 -2 Na2O1 Na O 1) CHARGE OF M & NM? 2) CROSS CHARGES 3) REMOVE SIGNS; remove 1 4) Name pattern: Metal NM-ide Na2O Sodium Oxide
Impromptu Board Practice for M + NM 1) Given two elements provide formula and name 2) Given name provide formula
What is the formula and name of the binary formed from Ca and N ? A.CaN Nitrogen calcide B. Ca2N3 calcium nitride C. Ca3N2 calcium nitrogen D.Ca3N2 calcium nitride 25% 25% 25% 25% Ca2N3 calcium nitride Ca3N2 calcium nitride CaN Nitrogen calcide Ca3N2 calcium nitrogen
What is the formula and name for a binary formed from K and P A. K3P potassium phosphide B. KP3 potassium triphosphorus C. K3P potassium phosphorus D. K2P3 potassium phosphide 25% 25% 25% 25% K3P potassium phosphide K2P3 potassium phosphide K3P potassium phosphorus KP3 potassium triphosp...
Magnesium oxides formula is: A.MgO B. Mg2O C. MgO2 D.Mg3O2 25% 25% 25% 25% MgO MgO2 Mg3O2 Mg2O
Lithium arsenides formula is: A.LiAs3 B. Li3As C. LiAs D.Li3As2 25% 25% 25% 25% Li3As2 LiAs Li3As LiAs3
Binary compound naming 1. Non-Metal + Non-Metal 2. Metal + Non-Metal 3. Transition Metal + Non-Metal = NM+NM = M + NM = TM + NM
Building and Naming TM + NM +1,2,3,4, (depends) With TM you can t predict the charge. It has to be a given.
Building and Naming TM + NM GIVEN +3 -2 Fe-2O+3 O Fe 1) CHARGE of NM? 2) CROSS CHARGES 3) REMOVE SIGNS; 4) Name pattern: TM (given charge) NM-ide Fe2O3 Iron(III) Oxide Use Roman numerals for charge
Impromptu Board Practice for TM + NM 1) Given two elements and TM charge provide formula and name 2) Given name provide formula
Given Mn is +5, what is the correct formula and name when it is combined with N? A. Mn3N5, magnesium nitride B. Mn5N3. manganese(5) nitride C. Mn3N5 manganese (V) nitride D. Mn3N5 manganese nitride 25% 25% 25% 25% Mn3N5, magnesium nitride Mn3N5 manganese nitride Mn5N3. manganese(5) ni... Mn3N5 manganese (V) n...
Given that Fe is +2, what is the formula and name of compound formed with Cl A.FeCl3, iron chloride B. Fe3Cl iron(III) chloride C. FeCl2 iron(I) chloride D.FeCl2, iron(II) chloride 25% 25% 25% 25% FeCl3, iron chloride FeCl2 iron(I) chloride FeCl2, iron(II) chloride Fe3Cl iron(III) chloride
What is the formula for Nickel(III) oxide ? A.Ni2O3 B.Ni3O2 C. Ni3O D.NiO 25% 25% 25% 25% NiO Ni3O Ni2O3 Ni3O2
Binary compound naming 1. Non-Metal + Non-Metal 2. Metal + Non-Metal 3. Transition Metal + Non-Metal = NM+NM = M + NM = TM + NM
Building and Naming NM + NM NM + NM combos don t involve charge! NM + NM combos must be given, not predicted Many different NM +NM combos are possible for any two NM Example: N + O combos N2O, NO2, N2O5 , NO , N2O4
Building and Naming NM + NM(cont.) To indicate element count for NM + NM use Table 2.6 page 67 of text Atom count prefix 1 mono 2 di 3 tri 4 tetra 5 penta 6 hexa 7 hepta 8 octa 9 nona 10 deca
Building and Naming NM + NM (cont.) Examples of using the prefixes to name NM +NM binaries N2O4 Dinitrogen tetraoxide Dihydrogen monoxide H2O Mononitrogen monoxide NO nitrogen monoxide
Impromptu Board Practice for NM + NM 1) Given two elements and atom counts provide name 2) Given name provide formula