C-WORTHY Study: Grazoprevir/Elbasvir Treatment in HCV Genotype 1
C-WORTHY Study Part B compares 12-week vs. 18-week treatment of grazoprevir/elbasvir plus ribavirin in HCV genotype 1 patients. The study includes treatment-naive individuals with cirrhosis and null responders with or without cirrhosis. Baseline characteristics, patient disposition, dosage of study drugs, and outcomes are reported. Primary efficacy endpoint is sustained virologic response at 12 weeks post-treatment initiation. Detailed data on patient demographics, HCV RNA levels, adverse events, and treatment discontinuations are presented. The study is significant for evaluating the efficacy and safety of this treatment regimen.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
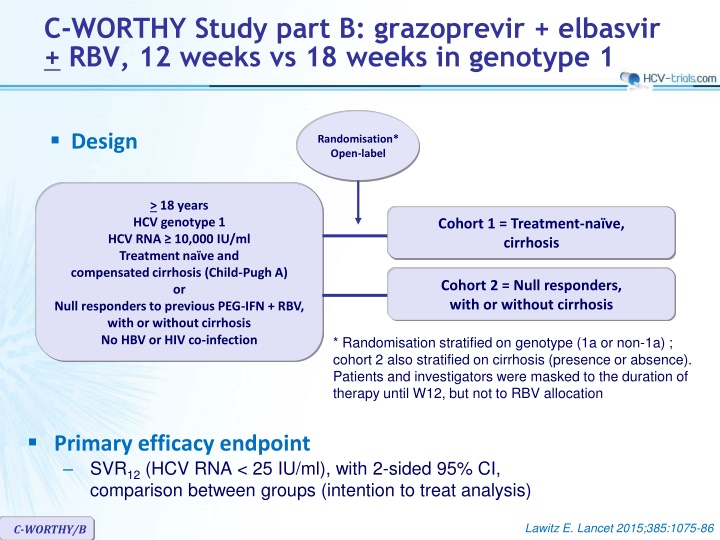
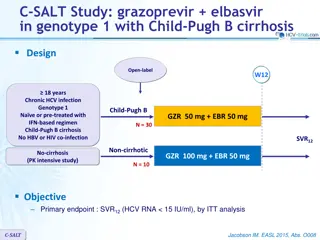
C-WORTHY Study part B: grazoprevir + elbasvir + RBV, 12 weeks vs 18 weeks in genotype 1 Design Randomisation* Open-label > 18 years HCV genotype 1 HCV RNA 10,000 IU/ml Treatment na ve and compensated cirrhosis (Child-Pugh A) or Null responders to previous PEG-IFN + RBV, with or without cirrhosis No HBV or HIV co-infection Cohort 1 = Treatment-na ve, cirrhosis Cohort 2 = Null responders, with or without cirrhosis * Randomisation stratified on genotype (1a or non-1a) ; cohort 2 also stratified on cirrhosis (presence or absence). Patients and investigators were masked to the duration of therapy until W12, but not to RBV allocation Primary efficacy endpoint SVR12(HCV RNA < 25 IU/ml), with 2-sided 95% CI, comparison between groups (intention to treat analysis) Lawitz E. Lancet 2015;385:1075-86 C-WORTHY/B
C-WORTHY Study part B: grazoprevir + elbasvir + RBV, 12 weeks vs 18 weeks in genotype 1 Treatment groups W18 W12 N = 31 GZR + EBR + RBV = B4 Cohort 1 N = 29 GZR + EBR = B5 Na ve with cirrhosis N = 32 GZR + EBR + RBV = B6 N = 31 GZR + EBR = B7 N = 32 GZR + EBR + RBV = B8 Cohort 2 GZR + EBR = B9 N = 33 Null responders to PEG-IFN + RBV with or without cirrhosis N = 33 GZR + EBR + RBV = B10 GZR + EBR = B11 N = 32 Dosage of study drugs Grazoprevir (GZR) 100 mg qd Elbasvir (EBR) : 50 mg qd RBV (bid dosing) : 800mg/day if 51-65 kg, 1000 mg/day if 66-80 kg, 1200 mg/day if 81-105 kg, 1400 mg/day if > 105 kg C-WORTHY/B Lawitz E. Lancet 2015;385:1075-86
C-WORTHY Study part B: grazoprevir + elbasvir + RBV, 12 weeks vs 18 weeks in genotype 1 Baseline characteristics and patient disposition (Cohort 1 : treatment-na ve with cirrhosis) GZR + EBR + RBV 12W N = 31 GZR + EBR 12W N = 29 GZR + EBR + RBV 18W N = 32 GZR + EBR 18W N = 31 Mean age, years 57 59 59 59 Female 39% 34% 53% 32% HCV genotype 1a 1b other 75% 25% 0 74% 26% 0 65% 32% 3% 69% 24% 7% HCV RNA log10IU/ml, mean 6.53 6.43 6.4 6.6 Discontinued treatment , N Virologic failure Adverse event 3 3 0 1 1 0 1 0 1 1 1 0 Lawitz E. Lancet 2015;385:1075-86 C-WORTHY/B
C-WORTHY Study part B: grazoprevir + elbasvir + RBV, 12 weeks vs 18 weeks in genotype 1 Baseline characteristics and patient disposition (Cohort 2 : null responders with or without cirrhosis) GZR + EBR + RBV 12W N = 32 GZR + EBR 12W N = 33 GZR + EBR + RBV 18W N = 33 GZR + EBR 18W N = 32 Mean age, years 52 54 56 54 Female 37% 39% 52% 44% HCV genotype 1a 1b 56% 44% 67% 33% 58% 42% 53% 47% HCV RNA log10IU/ml, mean 6.64 6.67 6.81 6.80 Discontinued treatment , N Virologic failure Adverse event Death 2 0 1 1 3 3 0 0 1 0 0 0 1 1 0 0 Lawitz E. Lancet 2015;385:1075-86 C-WORTHY/B
C-WORTHY Study part B: grazoprevir + elbasvir + RBV, 12 weeks vs 18 weeks in genotype 1 SVR12(HCV RNA < 25 IU/ml) , % (95% CI) GZR + EBR + RBV 12W GZR + EBR 12W GZR + EBR + RBV 18W GZR + EBR 18W % 100 97 97 97 94 91 94 (89-100) 90 (84-100) (84-100) 100 (82-100) (79-99) (76-98) (79-99) (74-88) 80 60 40 20 N 31 29 32 31 32 33 33 32 0 Treatment-na ve (Cohort 1) Null responders (Cohort 2) Early discontinuation 0 0 1 0 2 0 0 0 Virologic breakthrough 1 0 0 0 0 0 0 1 Relapse 2 1 0 2 0 3 0 0 Cohort 2 with cirrhosis : SVR12= 92% with 12W and 100% with 18W ; 94% for GT1a and 100% for GT1b Lawitz E. Lancet 2015;385:1075-86 C-WORTHY/B
C-WORTHY Study part B: grazoprevir + elbasvir + RBV, 12 weeks vs 18 weeks in genotype 1 Adverse events and laboratory abnormalities, N (%) GZR + EBR + RBV 12W GZR + EBR 12W GZR + EBR + RBV 18W GZR + EBR 18W Serious adverse events 2 (3%) 3 (5%) 1 (2%) 2 (3%) Discontinuation due to AE 1 (2%) 0 1 (2%) 0 Death 1 (2%) 0 0 0 Adverse events in 10% Fatigue 24% 23% 37% 21% Headache 17% 18% 26% 32% Asthenia 14% 9% 16% 14% Hemoglobin < 10 g/dl 10% 0 8% 0 Elevation of bilirubin > 2.5 to 5.0 x baseline 11% 0 28% 6% Elevation of bilirubin > 5 x baseline 2 (3%) 0 1 (2%) 0 Elevation of ALT or AST > 2.5 x baseline 0 0 1 (2%) 5 (8%) Lawitz E. Lancet 2015;385:1075-86 C-WORTHY/B
C-WORTHY Study part B: grazoprevir + elbasvir + RBV, 12 weeks vs 18 weeks in genotype 1 SVR12according to detection of resistance-associated variants at baseline NS3 variants at baseline NS5A variants at baseline NO YES NO YES N 169 79 209 34 SVR12 (HCV RNA < 25 IU/ml) 96.4% 92.4% 97.1%* 82.4% * Most prevalent NS5A variants at failure : M28T Q30L/R L31M Y93H/N Most prevalent NS3 variants at failure : Y56H A156T/G/V D168A/Y Mutations detected * p < 0.001 Lawitz E. Lancet 2015;385:1075-86 C-WORTHY/B
C-WORTHY Study part B: grazoprevir + elbasvir + RBV, 12 weeks vs 18 weeks in genotype 1 Summary In this phase II study, oral treatment with grazoprevir and elbasvir, with or without RBV, in HCV genotype 1-infected patients that are difficult to cure with HCV therapy (patients with well compensated cirrhosis and null responders with or without well compensated cirrhosis), high rates of SVR12 were shown across all groups, irrespective of the addition of RBV or extension of treatment duration from 12 to 18 weeks 12 weeks of GZR + EBR without RBV achieved SVR12 of 97% in previously untreated patients with cirrhosis, 91% in null responder patients with or without cirrhosis, 92% in null responder patients with cirrhosis The rate of virologic failure with GZR + EBR with or without RBV was low (4%) Similar efficacy was seen in patients with genotype 1a and 1b Patients with NS5A baseline resistance-associated variants had lower SVR12 Treatment-emergent, clinically significant, adverse events were infrequent No discontinuation for AE in the groups without RBV Lawitz E. Lancet 2015;385:1075-86 C-WORTHY/B