Calculate Gas Balloon Volume at STP from Given Conditions
Learn how to determine the volume of a gas-filled balloon at standard temperature and pressure (STP) when given the initial volume, temperature, and pressure. In this case, the initial volume is 30.0 L at a temperature of 313 K and pressure of 153 kPa. By applying the ideal gas law and standard conditions of 273 K and 101.3 kPa, you can find the final volume at STP. Follow the provided steps to accurately calculate the volume at standard conditions.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
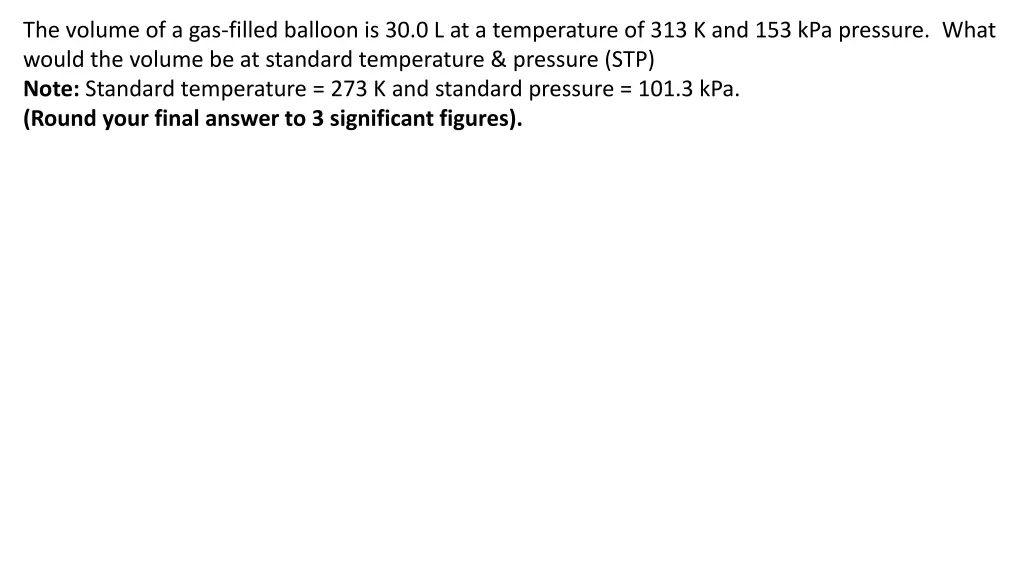
The volume of a gas-filled balloon is 30.0 L at a temperature of 313 K and 153 kPa pressure. What would the volume be at standard temperature & pressure (STP) Note: Standard temperature = 273 K and standard pressure = 101.3 kPa. (Round your final answer to 3 significant figures).
The volume of a gas-filled balloon is 30.0 L at a temperature of 313 K and 153 kPa pressure. What would the volume be at standard temperature & pressure (STP) Note: Standard temperature = 273 K and standard pressure = 101.3 kPa. (Round your final answer to 3 significant figures).
The volume of a gas-filled balloon is 30.0 L at a temperature of 313 K and 153 kPa pressure. What would the volume be at standard temperature & pressure (STP) Note: Standard temperature = 273 K and standard pressure = 101.3 kPa. (Round your final answer to 3 significant figures).
The volume of a gas-filled balloon is 30.0 L at a temperature of 313 K and 153 kPa pressure. What would the volume be at standard temperature & pressure (STP) Note: Standard temperature = 273 K and standard pressure = 101.3 kPa. (Round your final answer to 3 significant figures).
The volume of a gas-filled balloon is 30.0 L at a temperature of 313 K and 153 kPa pressure. What would the volume be at standard temperature & pressure (STP) Note: Standard temperature = 273 K and standard pressure = 101.3 kPa. (Round your final answer to 3 significant figures).
The volume of a gas-filled balloon is 30.0 L at a temperature of 313 K and 153 kPa pressure. What would the volume be at standard temperature & pressure (STP) Note: Standard temperature = 273 K and standard pressure = 101.3 kPa. (Round your final answer to 3 significant figures).