Carbohydrates and Monosaccharides in Biology
Explore the significance of carbohydrates in living organisms, including their structure and functions. Learn about monosaccharides such as glucose, fructose, and galactose, and their molecular compositions.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
BIO.A.2.2.1: To explain how carbon is uniquely suited to form biological macromolecules Objective: To discuss the unique properties of carbon and why carbohydrates are essential to living things Warmup: What kinds of materials do plants take in on a daily basis? sunlight H2O + CO2 C6H12O6 + O2
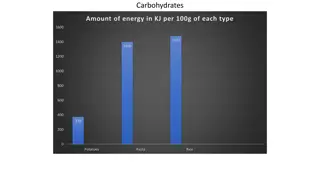
Made of saccharides (simple sugar) molecules Contain a ratio of: 1 carbon: 2 hydrogen: 1 oxygen (example: C6H12O6) Carbohydrates release lots of energy when the bonds within them are broken. For example: C6 H12O6 Bond breaks = Energy
Most dissolve easily in water, therefore are hydrophilic Functions: 1) Energy source 2) Make up organelles (ex. cell walls, cell membranes)
Monosaccharidesone simple sugar unit Examples: glucose fructose galactose 1. (C6H12O6) (C6H12O6) (C6H12O6) Molecules (see above) with the same formula but different structural arrangements are called isomers (see below)
Monosaccharide characteristics: fast acting (break bonds easily) regulated by insulin Questions: A) What three different types of atoms, or elements, are present in the monosaccharides shown previously? Carbon, hydrogen, oxygen
Questions: B) Write the molecular formula for glucose, fructose and galactose by adding the subscripts to the following: Glucose: Fructose: Galactose: C6H12O6 C6H12O6 C6H12O6
Questions: C) Compare the number of hydrogen atoms to the number of oxygen atoms in each sugar. What is the ratio of hydrogen to oxygen? 2:1
Questions: D) Cut out a model of glucose and glue it in the box below. Glucose
Questions: E) What atoms are attached to each end of the glucose molecule? OH, H
Disaccharidetwo simple sugar units Examples: sucrose maltose lactose 2. (C12H12O11) (C12H22O11) (C12H22O11) Disaccharide characteristics: fast acting (break easily) slightly larger than monosaccharides
Questions: A) Cut out a model of glucose and fructose along the solid lines only. Try to join the two molecules together to form a disaccharide called sucrose. B) Will the two models stay together to form one model of sucrose? No
Questions: C) In order to join them together, you must remove an OH end from one molecule and an H from the other. Cut along the dotted lines. D) Glue the joined models together in the box below. Attach the OH and H ends and glue that below as well. Label the name of the new molecule.
Questions: E) Cut out a model of glucose and galactose to form the disaccharide lactose. Glue the joined models together in the box below. Attach the OH and H ends and glue that below as well. Label the names of the two new molecules. F)
BIO.A.2.2.1: To explain how carbon is uniquely suited to form biological macromolecules Objective: To discuss the unique properties of carbon and why carbohydrates are essential to living things Warmup: 1.) Sugars such as glucose, fructose and ribose are examples of . a. Nucleic acids b. Carbohydrates c. lipids d. Proteins 2.) Carbon atoms have shell. One carbon atom can form with other atoms. electrons in their outer bonds
Questions: G) Write the molecular formula for lactose by adding the correct subscripts. Use the structural formulas as a guide and remember that one water was lost. Lactose: C12H22O11 H) Determine the ratios of hydrogen atoms to oxygen atoms for: Sucrose and lactose: Glucose and fructose: H 2 O 1 H 2 O 1
Questions: How many monosaccharide molecules are needed to construct a disaccharide molecule? 2 I)
Polysaccharidemany (100s-1000s) sugar units Examples: starch found in plants (for energy) glycogen found in animals (for energy) cellulose found in plants (for support) chitin found in animals (for support) 3. Polysaccharide characteristics: long lasting energy source (many bonds)
Questions: A) Construct a starch molecule by joining three glucose molecules. This will only represent a small part of a starch molecule. B) Attach the molecule in the area below and title them. There should be one polysaccharide molecule and two water molecules.
Questions: C) What smaller molecules make up all polysaccharides? monosaccharides D) Mono means one, di means two and poly means many. Why are these prefixes used in describing the three main types of carbohydrates? Describes the number of monomers in the larger polymer.
Questions: E) Synthesis means putting together. Dehydrate means loss of water. Explain why biologists refer to the joining of monosaccharide molecules as a dehydration synthesis, or condensation, reaction. Water is removed when monomers (monosaccharides) are joined together to make a larger polymer (di- or polysaccharide)