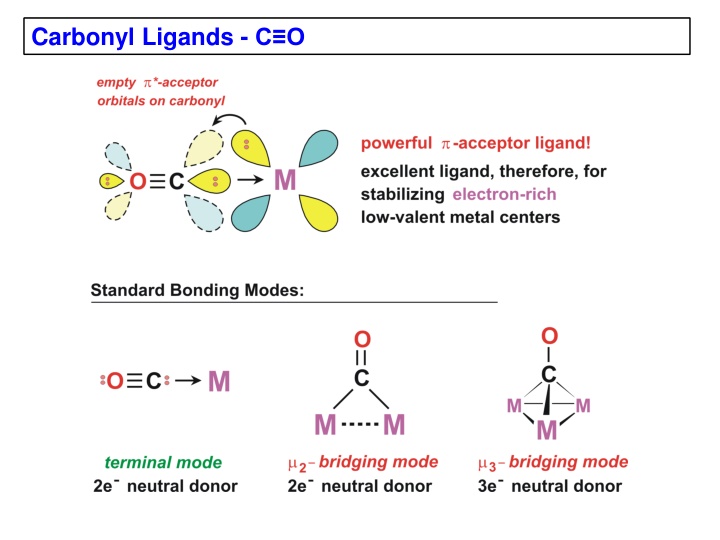
Carbonyl Ligands -
Metal-carbonyl complexes are important in coordination chemistry. This content covers examples of neutral binary metal carbonyls, MO diagrams, CO-metal bonding interactions, IR stretching frequencies, and electronic effects on CO. The text explores the nature of metal-CO bonding, the positioning of carbonyl bands in IR spectra, and the impact of electron density on CO backbonding. Explore the intricacies of M-C and C-O bonds, the effects of electron density on metal centers, and the corresponding vibrational frequencies observed in IR spectroscopy.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Examples of neutral, binary metal carbonyls: 4 5 6 7 8 9 10 11 Fe(CO)5 Fe2(CO)9 Fe3(CO)12 Co2(CO)8 Co4(CO)12 Ti V(CO)6 Cr(CO)6 Mn2(CO)10 Ni(CO)4 Cu Nb Mo(CO)6 Tc2(CO)10 Ru(CO)5 Ru3(CO)12 Rh4(CO)12 Rh6(CO)16 Zr Pd Ag Hf Ta W(CO)6 Re2(CO)10 Os(CO)5 Os3(CO)12 Ir4(CO)12 Pt Au
Molecular Orbital (MO) Diagram Experimental Data Supporting Nature of MO s in CO CO cm-1 2143 2184 1489 1715 Species CO Config (5 )2 (5 )1 (5 )1(2 )1 C-O 1.13 1.11 S 1.24 T 1.21 Comment 5 MO is weakly antibonding 2 MO is strongly antibonding CO*
Three types (two of which are important) of CO-Metal bonding interactions: M-C bond: C-O bond: CO freq: increases increases increases increases decreases decreases increases decreases decreases
Carbonyl Infrared (IR) Stretching Frequencies The position of the carbonyl bands in the IR depends mainly on the bonding mode of the CO (terminal, bridging) and the amount of electron density on the metal being -backbonded to the CO. The number (and intensity) of the carbonyl bands observed depends on the number of CO ligands present and the symmetry of the metal complex. There are also secondary effects such as Fermi resonance and overtone interactions that can complicate carbonyl IR spectra. O O C M C O C O C M M M 2 M M bridging 3 bridging terminal mode free CO COIR(cm ) -1 2143 2120 - 1850 1850 - 1720 1730 - 1500 (for neutral metal complexes)
Electronic Effects on CO As the electron density on a metal center increases, more - backbonding to the CO ligand(s) takes place. This further weakens the C-O bond by pumping more electron density into the formally empty carbonyl * orbital. This increases the M-CO bond strength making it more double-bond-like, i.e., the resonance structure M=C=O assumes more importance. d x CO cm 1 Complex free CO 2143 d10 [Ag(CO)]+ 2204 Ni(CO)4 2060 [Co(CO)4] 1890 [Fe(CO)4]2 1790 [Mn(CO)6]+ 2090 d6 Cr(CO)6 2000 [V(CO)6] 1860
Ph2 Ph2 P P 2- Ph2 O O O CO C P Fe Fe C C C C O O Fe Fe C O C O C CO O C C P Ph2 O O 2100 2000 1900 1800 -1 Wavenumbers (cm )
O O O C C C Ni ( -CO)(CO) (dppm) 2 Ni Ni 2 2 P P P P -CO +CO P P Ni (CO) (dppm) 2 4 CO 2 O C Ni Ni C C O O P P -CO +CO P P Ni(CO) ( -dppm) 3 O 1 2 C Ni C O C O 2000 1900 1800 1700 -1 Wavenumbers (cm )
Ligand Electronic Effects on CO CO cm 1 Complex Mo(CO)3(PF3)3 Mo(CO)3(PCl3)3 Mo(CO)3[P(OMe)3]3 Mo(CO)3(PPh3)3 Mo(CO)3(NCCH3)3 Mo(CO)3(triamine)3 Mo(CO)3(pyridine)3 2090, 2055 2040, 1991 1977, 1888 1934, 1835 1915, 1783 1898, 1758 1888, 1746 Based on CO IR stretching frequencies, the following ligands can be ranked from best -acceptor to worst: NO+ > CO > PF3 > RN C > PCl3 > P(OR)3 > PR3 > RC N > NH3
Semi-Bridging Carbonyls O Unsymmetrical bridging form. * system accepts electron density from second metal center. Distortions away from a linear M-CO (180 ) or a symmetrically bridging CO (120 ). Typical M-CO angle around 150 (but with considerable variations). ~ 150 C M M filled Fe d orbital O O O C C C Cotton & Troup JACS, 1974, 96, 1233 Fe Fe C O N C C N O O CO * empty antibonding acceptor orbital
/ Bridging COs O O O C C C M M M M M M M M CO acting as -donor or -acceptor? 1.30 2.22 O 2.25 O C C O 1.97 C Nb Nb Cp Cp Nb C C O O C Cp C O O Herrman & coworkers JACS, 1981, 103, 1692
Problem: Which of the following metal carbonyl IR spectra represents the compound with the least amount of electron density on the metal center? Briefly discuss the reasoning for your choice. Which compound will lose CO the easiest?
Problem: Which of the following metal carbonyl compounds will have the highest CO stretching frequency in the IR? Why? Will this be the most electron-rich or deficient compound? a) b) CO CO F CO Br CO Ir Ir F CO Br CO F Br c) CO Me2N CO Ir Me2N CO NMe2