Cell Notation and Diagrams for Redox Reactions
Explore various redox reactions through detailed cell notations and diagrams, including components of anode and cathode compartments, oxidation and reduction half-cells, salt bridge usage, and overall reactions. Visual representations provided for better understanding.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
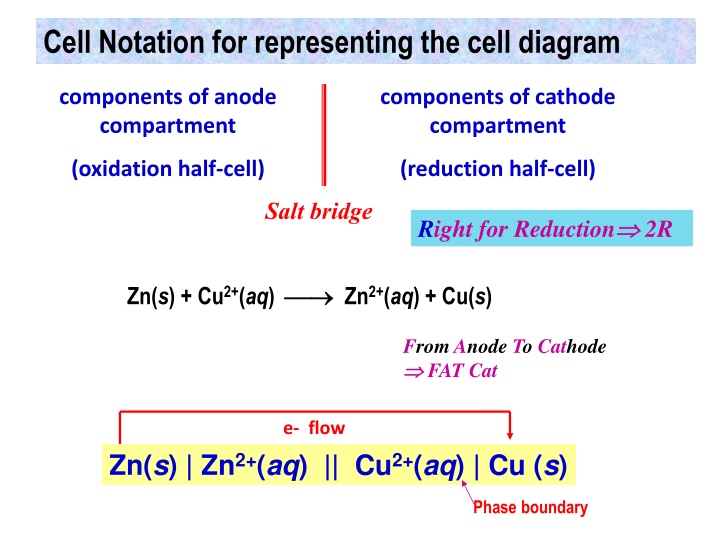
Cell Notation for representing the cell diagram components of anode compartment components of cathode compartment (oxidation half-cell) (reduction half-cell) Salt bridge Right for Reduction 2R Zn(s) + Cu2+(aq) Zn2+(aq) + Cu(s) From Anode To Cathode FAT Cat e- flow Zn(s) | Zn2+(aq) || Cu2+(aq) | Cu (s) Phase boundary
2 Anode (Ox) : Cathode (Red) : Cr(s) Cr3+(aq) + 3e- 3 Cu2+(aq) + 2e- Cu(s) Overall reaction: 2Cr(s) + 3Cu2+(aq) 2Cr3+(aq) + 3Cu(s) Cell Diagram e- V salt bridge Cathode (+) Anode (-) Cr(s) Cu(s) Cu2+(aq) Cr3+(aq) Cr(s) | Cr3+(aq) || Cu2+(aq) | Cu (s) Cell Notation
2MnO4-(aq) + 16H+(aq) + 10I-(aq) 2Mn2+(aq) + 5I2(s) + 8H2O(l) Reduction half-reaction MnO4-(aq) + 8H+(aq) + 5e- Oxidation half-reaction 2I-(aq) I2(s) + 2e- Mn2+(aq) + 4H2O(l) Cell Diagram Cell Notation graphite (s) | I-(aq) | I2(s) || H+(aq), MnO4-(aq) , Mn2+(aq) | graphite (s) inert electrode
2 Anode (Ox) : Cathode (Red) : Fe3+(aq) Fe3+(aq) + e- Cl2 (g) + 2e- 2Cl- (aq) Overall reaction: 2 Fe3+(aq) + Cl2 (g) 2Fe3+(aq) + 2Cl- (aq) Cell Diagram e- V salt bridge Cathode (+) Anode (-) Pt Eletrode Cl2(g) at 1 atm, 25 oC Fe3+(aq) 1 M Pt Electrode Cl- (aq) 1 M Cell Notation Fe2+(aq) 1 M Pt (s) | Fe2+(aq, 1 M),Fe3+(aq, 1 M) || Cl2(g, 1 atm) | Cl- (aq, 1 M) | Pt (s)