Chemical Analysis and Chromatography Techniques: Understanding Pure and Impure Substances
Explore the distinctions between pure and impure substances in chemical analysis, learning about their properties and uses. Dive into gas tests for identifying hydrogen, oxygen, chlorine, and more. Delve into the world of chromatography and understand how it separates substances based on solubilities. Enhance your knowledge with practical examples and conclusions from chromatography exams.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
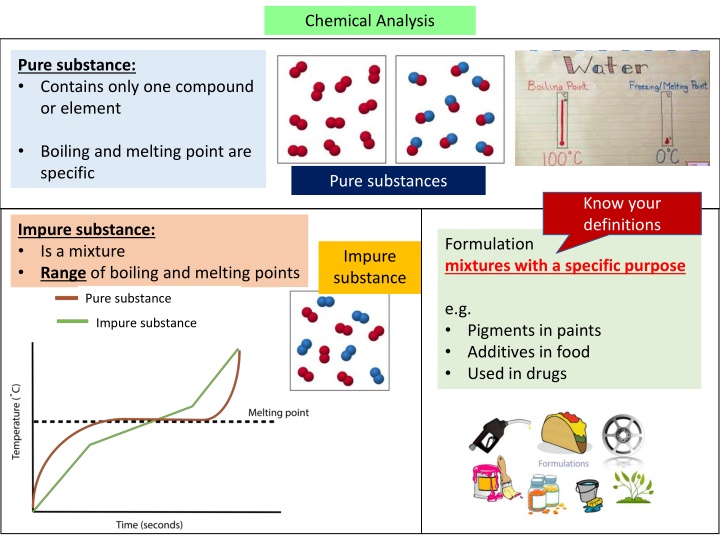
Chemical Analysis Pure substance: Contains only one compound or element Boiling and melting point are specific Pure substances Know your definitions Impure substance: Is a mixture Range of boiling and melting points Formulation mixtures with a specific purpose Impure substance Pure substance e.g. Impure substance Pigments in paints Additives in food Used in drugs
Gas tests Hydrogen Oxygen Lit split will burn with a squeaky pop Relights a glowing splint Chlorine Carbon dioxide Limewater turns cloudy Damp litmus paper bleaches white
Chromatography RP 1. Draw a pencil line 2cm from the bottom of the paper pencil will not run 2. Add dye / unknown substance as a small dot on the line stationary phase 3. Place the paper into a solvent ensure solvent below the line Rf must be less than 1 4. Let the solvent run up the paper if substances in the mixture are soluble they will run up the paper mobile phase Example 5. When the solvent is 1cm from the top of the paper remove and draw a pencil line solvent front
Chromatography exam question Conclusions Black is a mixture more than 1 colour dot in a column Black contains red and blue as dots correspond with same distance of known blue and red Black also contains an unknown - if no other knows match must state it is an unknown Chromatography separates substances in a mixture by their different solubilities To identify what is in a sample, run known samples against the unknown, on the same chromatogram