Chemical Bonding and Ionic Compounds
This material covers topics such as matter, atomic structure, bonding, and predicting chemical formulas in ionic compounds. Students will learn about valence electrons, naming binary ionic compounds, and the rules for forming correct chemical formulas. The content emphasizes the importance of achieving stability through valence electron configuration. Detailed discussions, activities, and key questions enhance understanding.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Living By Chemistry SECOND EDITION Unit 1: ALCHEMY Matter, Atomic Structure, and Bonding
Lesson 21: Salty Eights Formulas for Ionic Compounds
ChemCatalyst Find these cards in Your Salty Eights card deck. 1. List the ionic compounds you can make with pairs of cards, using two different elements. 2. List the ionic compounds you can make with three cards and only two different elements. 3. What rule must all these compounds satisfy?
Key Question How can you predict chemical formulas and name ionic compounds?
You will be able to: use valence electrons to predict ionic compounds develop proficiency at naming binary ionic compounds and writing their chemical formulas
Prepare for the Activity Work in groups of four.
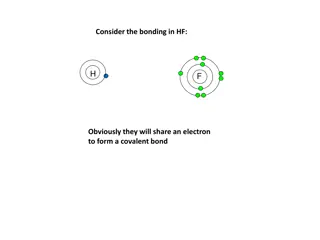
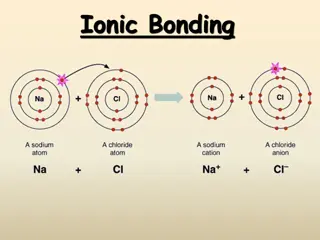
Discussion Notes In general, atoms come together to form an ionic compound if the number of valence electrons totals 8 or a multiple of 8.
Discussion Notes (cont.) Writing correct chemical formulas is a matter of keeping track of exactly how many atoms come together to make a compound. Creating correct chemical names is a matter of remembering some basic guidelines.
Wrap Up How can you predict chemical formulas and name ionic compounds? Ionic compounds tend to form from atoms that together have a total of 8 (or a multiple of 8) electrons in their outermost (valence) shells. Noble gases already have eight valence electrons and don t combine with other elements to make new compounds. They are already highly stable.
Check-In Which of these compounds are likely to form? a. Na2S d. Na3N b. K2Mg c. AlBr2 e. OCl f. CaO