Chemical Bonding and Particle Interaction
Understanding the structure and bonding in chemicals involves looking at the arrangement of particles and the forces that hold them together. By considering the number of electrons in the outer energy levels of atoms, we can determine how elements form compounds through the transfer of electrons between metals and non-metals, leading to the formation of ions and the attraction between positive and negative ions known as electrostatic attraction.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
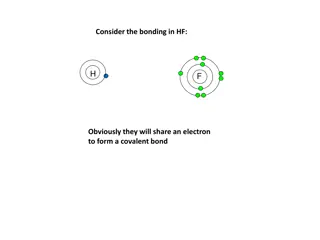
STRUCTURE & BONDING !!! What holds particles together, and what are the particles we have within chemicals
Lets start with remembering pattern from drawing atoms lesson (see previous work) Looking at the number of electrons atoms have in their outermost energy levels and linking this to their group number that they are positioned in. Group number 1 2 3 4 5 6 7 8 Number of electrons in outermost energy level 1 2 3 4 5 6 7 FULL
Group 8, DO NOT FORM COMPOUNDS! Why?? There is a stability associated with FULL OUTERMOST ENERGY LEVELS OF ELECTRONS, and this idea drives what can happen and what cannot in our chemical world. So METALS, which only have a few electrons will LOSE their outermost electrons, ending up with full energy levels. This is the reason why metals behave as metals NON-METALS, which need only a few more electrons to get full energy levels will GAIN electrons to fill up their outermost energy levels. This gaining of electrons gives non-metals their chemical properties Compounds are made from elements....
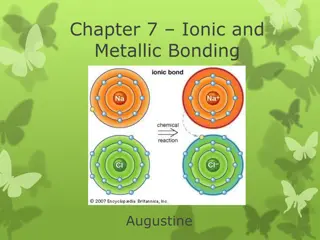
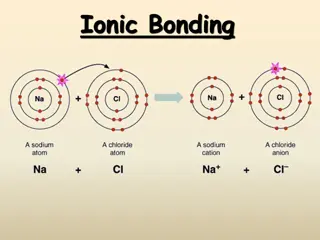
So consider a metal element like sodium, bonding with a non- metal element like chlorine Then the sodium atoms would all lose one electron, and all these lost electrons would fill up the chlorine atoms, like this.... IONIC BONDING = metal + non- metal
Atoms are neutral. When they lose electrons ( electrons are negatively charged) then the atoms become positively charged themselves. So metals lose electrons and become positively charged. Then the original ATOMS will become IONS
Similarly the non-metal atoms gain electrons (electrons are negatively charged) so the they become IONS and this time their charge will be negative. So non-metals form negative charged ions. Chlorine in this example would therefore form chloride ions like this..... Non metals form NEGATIVE ions
So when metal elements bond with non metal elements, there will be electrons lost by the metal atoms, and these electrons are gained by the non-metal element's atoms. This ELECTRON TRANSFER by lots of atoms will result in lots of IONS being formed. Positive and negative ions, which will naturally ATTRACT each other. This ELECTROSTATIC ATTRACTION BETWEEN POSITIVE AND NEGATIVE IONS IS IONIC BONDING. This attraction is strong and holds the ions into their positions. So let's look at the structure of the solid, sodium chloride which our famous ionic compound example. IONIC BONDING
Whenever we have a metal / non-metal combination of elements we will get this ionic bonding happening. The positive ions are next to negative ions, they ALTERNATE, within a huge repeating 3D pattern which we call a lattice You need to be able to draw, NaCl, structure You need to be able to extend these ideas to any examples they give you in exams So with my poor drawing skills lets see how I draw Na Cl structure.... STRONG ionic bonds (attractions)
First in pencil draw a cube shape Cube....
Then add the Chloride ions Middle of each of the three visible faces and at each corner that is visible, add a circle
Then add sodium ions, between the Cl- ions One smaller blob between the circles
Add a key/legend Larger circles should be Cl- Smaller blobs should be Na+
SOME properties of compounds When you understand the structure of compounds you need to be able to link this to the properties we observe for those chemicals in our world. Three properties exams about frequently are Whether the chemical will CONDUCT ELECTRICITY. Chemicals ONLY EVER conduct electricity if they have EITHER free ions, or free electrons moving about randomly within their structures. Whether the chemical has high or low melting and boiling points. These will be high if the particles have STRONG attractions and low if the attractions are weak Whether the chemicals dissolve in water. Chemicals will dissolve if they break up into their particles when added to water, otherwise they will stay undissolved
Properties of IONIC compounds HIGH boiling and melting points. Due to STRONG electrostatic attractions between positive and negative ions. Therefore, a high temperature will be needed to overcome these if the chemical is to melt or boil. Consequently the ionic chemicals are all solids at normal temperatures, with HIGH bpts and mpts. SOLIDS DO NOT CONDUCT ELECTRICITY. All the electrons are firmly held within energy levels, so they are not free. Also the ions are firmly held with strong electrostatic attractions in their places in solid ionic compounds. So no conduction is possible IONIC GASES, LIQUIDS and SOLUTIONS will all conduct electricity as the IONS will be free to move about. These free ions will be responsible for electrical conduction Many ionic compounds dissolve in water, but some do not. Water will often break down the lattice structure and the ions will then move freely within the water, and we see the solid dissolving. However if the electrostatic attractions are TOO strong, then water will not manage to break down the lattice structure and so some ionic compounds do not dissolve.
N:B Whenever we have a metal element bonding with a non metal element then we will get this ionic bonding happening. Ionic compounds are made up of particles known as IONS!!!! The ions attract each other strongly in a lattice, and their arrangements give their crystals a particular shape. (e.g. NaCl is cubic, look closely at your household salt crystals, they are tiny cubes!) Smallest of our crystals will contain many trillions of ions . Arguably, out of around 9 million chemicals in the world so far documented, about half a million of them are IONICALLY bonded. Next lesson, we discuss how the others are bonded .
Summary of Ionic bonding Happens when a METAL element bonds with a NON-METAL element Electron transfer takes place, metal atoms lose electrons and the non-metal atoms gain them. So positive metal ions are formed alongside negative non-metal ions. These ions attract each other, due to STRONG electrostatic attractions between positively charged ions and negatively charged ions formed. The ions become arranged into a crystal lattice which is a 3D giant repeating pattern, where the ions are held in their places by these strong ionic bonds Ions are arranged ALTERNATING +ve and ve ions, attractions outweigh the repulsions present within the lattice structure. Ionic bonds are simply electrostatic attractions between the +ve and ve ions present. Ionic compounds have high m.pts. and b.pts. Ionic compounds do not conduct electricity when SOLIDS Ionic compounds do conduct electricity when LIQUIDS or dissolved in water Many ionic compounds dissolve in water but some do not.