Chemical Bonding Principles
Delve into the fundamental concepts of chemical bonding, including bond energy, ionic bonding, Coulomb's Law, covalent bonding, electronegativity, and bond polarity. Explore how electrons interact, the role of bond length and strength, and the impact of electronegativity differences on bond types. Uncover the essence of bond polarity and dipole moments in molecules, shedding light on the intricate world of chemical interactions.
Uploaded on | 2 Views
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Bonding: General Concepts Dr. Walker DE Chemistry
Bonding Bond Energy Energy required to break a chemical bond Atoms bond in a way that achieves the lowest possible energy for the system Bond length distance between the nuclei of two bonded atoms Shorter bond length = stronger bond = higher bond energy!!
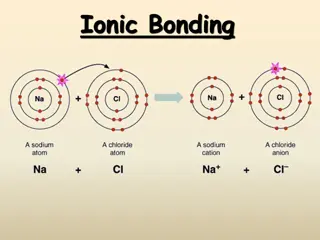
Ionic Bonding Electrons are transferred Metals react with nonmetals Ions paired together have lower energy (greater stability) than separated ions Ionic compounds conduct electricity in a molten state
Coulombs Law Shows energy of interaction between a pair of ions E = Energy, r = distance between ionic nuclei, Q = ionic charge Lower energy = stronger attraction, more favorable interaction
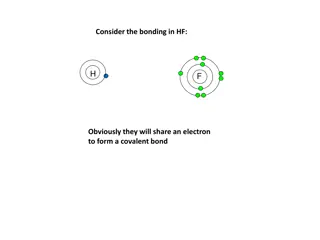
Covalent Bonding Electrons are shared by nuclei Pure covalent (non-polar covalent) Electrons are shared evenly Polar covalent bonds Electrons are shared unequally Atoms end up with fractional charges + or - (partial charges)
Electronegativity The ability of an atom in a molecule to attract shared electrons to itself. General Trend Increases to the right, Increases going up on the Periodic Table
Electronegativity and Bonding The greater the electronegativity difference, the greater the chance of a bond being ionic This has been quantified, but (as usual), there are exceptions.
Bond Polarity and Dipole Moments Dipolar Molecules Molecules with a somewhat negative end and a somewhat positive end (a dipole moment) due to a difference in electronegativity Molecules with preferential orientation in an electric field (see next slide) All diatomic molecules with a polar covalent bond are dipolar
Polar Bonds, Nonpolar Molecules Covalent molecules can have multiple dipole moments, but can be nonpolar overall due to symmetry.
Ions: Electron Configurations and Sizes Ionic bonds Electrons are transferred until each species attains a noble gas electron configuration (ns2np6) This trend is primarily for main group elements and still has exceptions (Pb2+, Sn2+ among others) Formulas are predicted based on atoms required to form a neutral atom Covalent bonds Electrons are shared in order to complete the valence configurations of both atoms Exceptions can occur below period 2 Note: This SHOULD be review. I m not sure why the book puts it here.
Ionic Size Anions are larger than the parent atom Cations are smaller than the parent atom Essentially, they lose a shell Ion size increases within a group (more energy levels) Isoelectronic ions Ions with the same number of electrons (for example, Cl-, S2-, and P3- all have 18 electrons) Size decreases as the nuclear charge (atomic number) increases
Energy In Ionic Compounds Lattice Energy The change in energy that takes place when separated gaseous ions are packed together to form an ionic solid Energy change is negative (- H) as it is released to the surroundings
Lattice Energy Modified form of Coulomb s Law, shows energy loss from lattice formation k = a proportionality constant dependent on the solid structure and the electron configuration Q1 and Q2are charges on the ions r = shortest distance between centers of the cations and the anions Lattice energy increases as the ionic charge increases and the distance between anions and cations decreases More charge, closer ions = more energy savings
Example Of Lattice Energy Lattice energy The final step of lattice formation is what makes the process exothermic overall
Crystal Lattices Our previous visualization of ionic salts as stand-alone compounds is NOT correct. Ionic salts form lattice structures, shown on the left. This network formation ultimately makes the energy work out in favor of bond creation. Ionic formulas are actually empirical formulas, since we don t count the atoms in an entire lattice, which is impossible
Bond Character Differences in electronegativity cause unequal sharing of electrons in covalent bonds (polarity) or complete transfer of electrons (ionic bonds) Some cases are ambiguous .it s hard to predict what they are Bond character most bonds are not 100% covalent or 100% ionic
Bond Character Bond Character measured by the above equation Ionic vs. Covalent Ionic compounds generally have greater than 50% ionic character Ionic compounds generally have electronegativity differences greater than 1.6 Percent ionic character is difficult to calculate for compounds containing polyatomic ions (each ion has covalent bonds)
Bonding As A Model Models are a way to represent what we observe in nature, especially in what we can t see with our eyes Models do not equal reality and are often wrong The way covalent bonds are represented are a model .. .these have proven to be a good representation in most cases, but some molecules require us to think of them as a unit rather than a collection of bonds Keep this in mind as we progress through the chapter
Covalent Bonding A Review Electron pairs are shared between two atoms in covalent bonding Single Bond one pair of electrons being shared Double Bond two pairs of electrons being shared Triple Bond three pairs of electrons being shared More electrons being shared = higher bond energy = shorter bond
Bond Energies For Covalent Bonds These bond energies are endothermic energy added to break the bonds Conversely, bond formation is exothermic (as previously mentioned)
Localized Electron Bonding Model Lone electron pairs Electrons localized on an atom (unshared) Bonding electron pairs Electrons found in the space between atoms (shared pairs) Localized Electron Model "A molecule is composed of atoms that are bound together by sharing pairs of electrons using the atomic orbitals of the bound atoms Lone Pair Bonding Pair
Localized Electron Bonding Model Derivations of the Localized Model Valence electron arrangement using Lewis structures Prediction of molecular geometry using VSEPR (valence shell electron pair repulsion) Description of the type of atomic orbitals used to share or hold lone pairs of electrons
Lewis Structures Lewis structures show how valence electrons are arranged among atoms in a molecule. Lewis structures reflect the central idea that stability of a compound relates to noble gas electron configuration (octet rule) Shared electrons pairs are covalent bonds and can be represented by two dots (:) or by a single line ( - )
HONC, HONC.. The HONC Rule Hydrogen (and Halogens) form one covalent bond Oxygen (and sulfur) form two covalent bonds One double bond, or two single bonds Nitrogen (and phosphorus) form three covalent bonds One triple bond, or three single bonds, or one double bond and a single bond Carbon (and silicon) form four covalent bonds. Two double bonds, or four single bonds, or a triple and a single, or a double and two singles
I Gotta Be MeExceptions To The Rules 2nd row elements C, N, O, F observe the octet rule (HONC rule as well). 2nd row elements B and Be often have fewer than 8 electrons around themselves - they are very reactive. 3rd row (like S and P, especially) and heavier elements CAN exceed the octet rule using empty valence d orbitals. When writing Lewis structures, satisfy octets first, then place electrons around elements having available d orbitals.
Completing a Lewis Structure -CH3Cl Make the atom wanting the most bonds central Add up available valence electrons: C = 4, H = (3)(1), Cl = 7 Total = 14 Join peripheral atoms to the central atom with electron pairs. H .. .. H Complete octets on atoms other than hydrogen with remaining electrons C .. Cl .. .. .. H
Multiple Covalent Bonds: Double bonds H H H H C C C C H H H H Ethene Two pairs of shared electrons
Multiple Covalent Bonds: Triple bonds H H C C C H H C Ethyne Three pairs of shared electrons
Drawing Lewis Structures This differs from what you learned In previous class, as I GREATLY simplified it. Step 1: Count the number of valence electrons in the compound Step 2: Connect the atoms around the central atom by single bonds Step 3: Place remaining electrons on the outside atoms to fulfill octet rule Step 4: Place any remaining electrons on the central atom. Step 5: If the central atom does not have an octet, share pairs of electrons as bonds from outside atoms.
The Problem With Lewis The nitrate ion shows two types of bonds: 1 double bond and 2 single bonds Experiments show ONLY ONE BOND TYPE with a bond length BETWEEN that of a single and double bond This is not the only molecule exhibiting this behavior Presents a limitation of Lewis structures and the Localized Electron Model
Resonance Resonance is invoked when more than one valid Lewis structure can be written for a particular molecule. H H Benzene, C6H6 H H H H H H H H H H The actual structure is an average of the resonance structures The bond lengths in the ring are identical and between those of single and double bonds
More Resonance Ozone (O3) Bond lengths are identical, between that of a single and double bond O O O Neither structure is Technically correct! O O O
Resonance in Polyatomic Ions Resonance in a carbonate ion: Resonance in an acetate ion:
Odd-Electron Molecules Lewis structure models work best with molecules with even numbers of total electrons The localized electron model is based on pairs, so it doesn t work so well with these Example NO, 11 total valence electrons
Formal Charge Method of handling ions/molecules with unusual arrangements to help determine proper Lewis structure
Assigning Formal Charge This pertains to a Lewis structure that you must draw first Number of valence electrons on the free atom minus thenumber of valence electrons assigned to the atom in the molecule Lone pair (unshared) electrons belong completely to the atom in question Shared electrons are divided equally between the sharing atoms The sum of the formal charges of all atoms in a given molecule or ion must equal the overall charge on that species If the charge on an ion is -2, the sum of the formal charges must be -2
Assigning Formal Charge In a molecule with many possibilities, the representation with the lowest formal charges is most appropriate Bottom choices are best here with no charge on Xenon higher than +1
Formal Charge Examples A) P = 0, O = 0, Cl = -1 B) S = 0, 2 oxygens = 0, 2 oxygens = -1 C) Cl = 0, 3 oxygens = 0, 1 oxygen = -1 D) P = 0, 1 oxygen = 0, 3 oxygens = -1
VSEPR Valence Shell Electron Pair Repulsion The structure around a given atom is determined principally by minimizing electron-pair repulsions. Translation: all atoms and electrons want their own personal space and get as far away from everyone else as possible
Possible Arrangements X + E 2 3 4 5 6 Overall Structure Linear Trigonal Planar Tetrahedral Trigonal bipyramidal Octahedral Forms AX2 AX3, AX2E AX4, AX3E, AX2E2 AX5, AX4E, AX3E2, AX2E3 AX6, AX5E, AX4E2 A = central atom X =atoms bonded to A E = nonbonding electron pairs on A
Linear Structure No unbonded electrons to deal with, chlorines as far apart as possible
Linear AX2 CO2