Chemical Bonds and Lewis Structures in Chemistry
Explore the significance of chemical bonds and Lewis structures in understanding how atoms transfer or share electrons, as elucidated by renowned chemists like Gilbert N. Lewis. Dive into the different types of compounds based on electron interactions and the octet rule, along with insights on Lewis dot formulas and valence electrons in covalent bonding.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
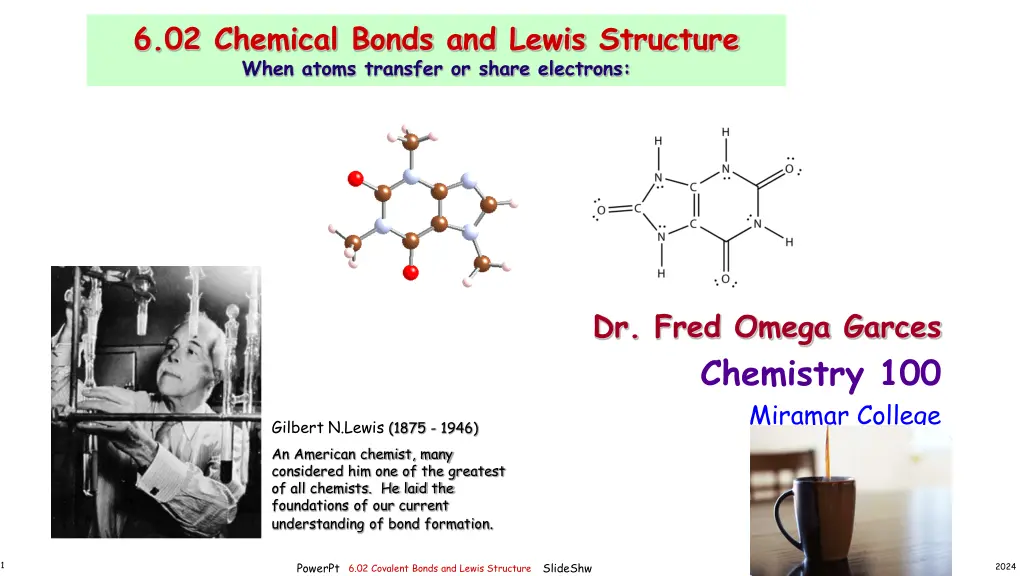
6.02 Chemical Bonds and Lewis Structure When atoms transfer or share electrons: Dr. Fred Omega Garces Chemistry 100 Miramar College Gilbert N.Lewis (1875 - 1946) An American chemist, many considered him one of the greatest of all chemists. He laid the foundations of our current understanding of bond formation. mage result for structure of caffeine gif 1 PowerPt SlideShw 2024 6.02 Covalent Bonds and Lewis Structure
Compound classification by electron interactions The diagram below shows how electrons interact between atoms to form compounds. When electrons are transferred, the compounds are ionic. The class of compounds called salts are in this category. When electrons are shared, covalent bonds are formed and this class of compound are called molecular compounds. When electrons are not evenly shared, the compounds are called polar covalent molecules. If the electrons from atoms making up the compound are mutually shared, these compounds are called Covalent Compounds. If the electrons from the atoms making up the compound are transferred between atoms, these compounds are called ionic compounds. If the electrons are unequally shared, then these are called polar covalent compounds. 2 2024 6.02 Covalent Bonds and Lewis Structure
Octet Rule Atoms and ions are most stable when they have complete outer shell filled with e-. (or when they have eight electrons in their valence shell). Exceptions: i. (Some compounds can accommodate more than 8 e- ) Elements in third period (especially transition metals) ii. (Some compounds will not lose or accept e-) Boron will not form B+3 because a small atom (B) can t stabilize the concentrate charge, likewise C and Si hardly forms +4 ions. Carbon, Silicon is not stable by donating or accept electrons. Instead, it is easier for these elements to share electrons. 3 2024 6.02 Covalent Bonds and Lewis Structure
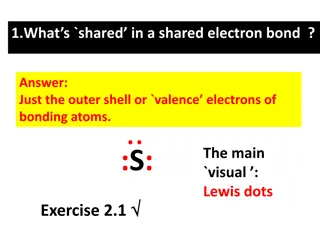
Lewis Dot Formulas of Molecules All elements in the periodic table possesses valence electron which can be represented by dots. The representation is known as "Lewis Dot Symbol. The Lewis Dot symbol for the selected elements are shown below. The valence electrons for each of these elements correspond to the group number above each family. VALENCE ELECTRONS 7 2024 6.02 Covalent Bonds and Lewis Structure
Valence electrons: Number of bonds Non-metallic elements tend to form covalent bonds. Covalent compounds are formed by nonmetallic elements. In general there is a pattern on how many bonds each element from of family will possess based on its valence electrons. The table below summarizes the number of covalent bonds from certain elements. In general, these are the number of bonds formed by these atoms. 8 2024 6.02 Covalent Bonds and Lewis Structure
Lewis Structure (Mostly for Molecular Compounds) ...according to the Octet Rule Using the Octet Rule, Molecular Lewis structure can be written. Molecular Lewis Structures are chemical representation showing how valence electrons are arranged in a chemical substance. When compounds are formed they tend to follow the Octet Rule (OR). Octet Rule: Atoms will share e- until it is surrounded by eight valence electrons. Rules of the game- i) Octet Rule works mostly for second period elements. Many exceptions especially with 3rd period elements (d-orbitals) ii) H prefers 2 e-, duet Rule, Only for Hydrogen through Beryllium (At.# 1 4) . . . .. . . . iii) :C: 4 u.p. 4 bonds O=C=O iv) H & F are terminal in the structural formula (Never central) N: 3u.p. 3 bonds N N :O: 2u.p. 2 bonds O = O :F: 1u.p. 1 bond F - F up = unpaired e- . 9 2024 6.02 Covalent Bonds and Lewis Structure
Lewis Structure by Periodic Group Examples of Lewis Structures according to Periodic Group Use Remaining Electrons to Achieve Noble Gas Configuration Calculate Number of Electrons Remaining Total Valence Electrons Draw Single Bonds Check Number of Electrons around element H, 2 F, 8 N,8 Compound H F a) HF 1 + 7 = 8 H-F 6 N N b) N2 5 + 5 = 10 N-N 8 c) NH3 5 + 3(1) = 8 2 N H H N H H H, 2 N, 8 H H H H H, 2 C, 8 d) CH4 4 + 4(1) = 8 0 C H H C H H H H F F, 8 C, 8 F e) CF4 4 + 4(7) = 32 24 C F F C F F F F N, 8 O, 8 + O N f) NO+ 5 + 6 - 1 = 10 N-O 8 10 2024 6.02 Covalent Bonds and Lewis Structure
Covalent Compound: Lewis Structure Consider the following chemicals- H C N F O Formaldehyde Difluoromoethyne Nitroglycerine 11 2024 6.02 Covalent Bonds and Lewis Structure
Covalent Compound: Lewis Structure Consider the following chemicals- Formaldehyde Difluoromoethyne Nitroglycerine 12 2024 6.02 Covalent Bonds and Lewis Structure
Lewis Structure dot structure via inspection Consider the following carbon dioxide: O C O Elements/Atomic sequence Lewis dot symbol Carbon dioxide Lewis Structure .. O C O.. Applying Octet rule 13 2024 6.02 Covalent Bonds and Lewis Structure
Lewis Structure by Bond Determination 1. (Connectivity) From the Chemical Formula, determine the atom connectivity for the structure. i. Given a chemical formula, ABn, A is the central atom and B flanks (surround) the A atom. i.e., NH3, NCl3, NO2. In these examples, N is central in the structure. ii. H and F are never central atoms. 2. (# of Bond) Determine the number of bonds in the compound, by calculating the theoretical Octet electrons (Oe) minus the total valence electrons (TVe). Oe is the theoretical number of electrons necessary for each atom in the structure to obtain a Noble Gas electron configuration, while TVe is the actual number of total valence electron for each atom in the structure. 3. (Remaining e-) Calculate the number of remaining electrons in the compound by taking the total valence electron (TVe) minus the number of electrons that was used to form bonds. Remaining e- divide by 2 = lone pairs. Complete Lewis structure by drawing atomic connectivity. Write bonds in the structure and place remaining electrons to selected atoms in the structure to give each atom an octet. Keep in mind that the H-atom is satisfied with 2 electrons (duet). 14 2024 6.02 Covalent Bonds and Lewis Structure
Lewis Dot Structure of CO2 by Bonds Table B. Calculate the number of bonds in compound structure. # bonds = (Oe - TVe) 2 bonds = (24- 16) = 2 A. Calculate Octet electrons (Oe-) and Total Valence electrons to determine number of bonds CO2 1 C 2 O Chg Oe 1 (8)= 8 2 (8)= 16 TVe 1 (4) = 4 2 (6)= 12 8 = 4 2 C. Calculate the remaining electrons to add to structure to complete Lewis dot structure. Remaining e- = TVe - e- used in bonding. = 16 - 8 = 8 e-Remaining 24 16 Writing Lewis Structure: First determine atom connectivity keeping in mind that H and F can never be central atoms. Generally when given the formula, ABn, A is the central atom in the structure (but not always), and B atoms flank the central atom. Next use information from the above calculations. Total of 16e- in CO2, of which 8 electrons are used to form 4 bonds and 8 remaining electrons are used to complete Lewis structure. .. .. O C O .. C O O .. O C O 1. Write atom connectivity for CO2. 2. Draw the four bonds in the structure. 3. Place the remaining 8 electrons in the structure to complete the Lewis Structure 15 2024 6.02 Covalent Bonds and Lewis Structure
Lewis Dot Structure of SO2 by Bonds Table A. Calculate (Oe-) and (TVe) B. Number of Bonds. SO2 1 S 2 O Oe 1 (8)= 8 2 (8)= 16 TV e- 1 (6) = 6 2 (6)= 12 # bonds = (24- 18) = 6 = 3 bonds 2 2 C. Remaining electrons. Remaining e- = 18 - 6 = 12 e- Remaining 24 18 Bonding Electrons (24- 18) = 6 D. Lone Pairs. Remaining e- / 2 = 12/2 = 6 LP Writing Lewis Structure: 3. Place the remaining 12 electrons in the structure such that each atom has an octet to complete the Lewis Structure 2. Draw the three bonds in the structure. 1. Write atom connectivity for SOs. S O O S O O 17 2024 6.02 Covalent Bonds and Lewis Structure
Lewis Dot Structure of ClO4- by Bonds Table A. Calculate (Oe-) and (TVe) ClO4- Oe 1 Cl 1 (8)= 8 4 O 4 (8)= 32 Chg 40 B. Number of Bonds. TV e 1 (7) = 7 4 (6)= 24 [ClO4-] 1 31 32 # bonds = (Oe- TVe) = # bonds 2 # bonds = (40- 32) = 8 = 4 bonds 2 2 C. Remaining electrons. Remaining e- = 32 - 8 = 24 e-Remaining 3. Place the remaining 24 electrons in the structure such that each atom has an octet to complete the Lewis Structure Writing Lewis Structure: 2. Draw the four bonds in the structure. 1. Write atom connectivity for ClO4-. O O O Cl O O O Cl O O Cl O O O O 18 2024 6.02 Covalent Bonds and Lewis Structure
Lewis Dot Structure of PO3-3 by Bonds Table A. Calculate (Oe-) and (TVe) B. Number of Bonds. PO3-3 Oe 1 P 1 (8)= 8 3 O 3 (8)= 24 Chg T Ve 1 (5) = 5 3 (6)= 18 3 26 # bonds = (Oe- TVe) = # bonds 2 # bonds = (32- 26) = 6 = 3 bonds 2 2 32 C. Remaining electrons. Remaining e- = 26 - 6 = 20 e-Remaining Writing Lewis Structure: 3. Place the remaining 20 electrons in the structure such that each atom has an octet to complete the Lewis Structure 1. Write atom connectivity for PO3-3. 2. Draw the three bonds in the structure. 3 O O O P O P O Cl P O O O O O 19 2024 6.02 Covalent Bonds and Lewis Structure
Lewis Dot Structure of H2PO3- by Bonds Table A. Calculate (Oe-) and (TVe) B. Number of Bonds. H2PO3- Oe 1 P 1 (8)= 8 3 O 3 (8)= 24 2 H 2 (2)= 4 2 (1)= 2 Chg 36 Tve 1 (5) = 5 3 (6)= 18 # bonds = (Oe- TVe) = # bonds 2 # bonds = (36- 26) = 10 = 5 bonds 2 2 1 26 C. Remaining electrons. Remaining e- = 26 - 10 = 16 e-Remaining Writing Lewis Structure: 3. Place the remaining 16 electrons in the structure such that each atom has an octet to complete the Lewis Structure 1. Write atom connectivity for H2PO3-. 2. Draw the five bonds in the structure. O O P O H P O H O H O H 20 2024 6.02 Covalent Bonds and Lewis Structure
Lewis Structures: Examples Example Cl i) CH2ClF ii) SiO2 O Si O F H C H 2- N O O iii) HNO3 iv) SO42- O H a) Linear b) bent (trigonal) c) trigonal d) tetrahedral e) pyramidal f) bent (tetrahedral) 21 2024 6.02 Covalent Bonds and Lewis Structure
Lewis Structures: Examples Example Cl i) CH2ClF ii) SiO2 O Si O F H C H 2- N O O iii) HNO3 iv) SO42- O H a) Linear b) bent (trigonal) c) trigonal d) tetrahedral e) pyramidal f) bent (tetrahedral) 22 2024 6.02 Covalent Bonds and Lewis Structure
Exception to Octet Rule There are generally three type of exception to the Octet Rule 1. Odd e- : Compounds with odd number of electrons (generally considered radicals which tend to be very reactive) 2. e- deficient : Compounds with a central atom with less than eight electrons in its valence shell. (Usually the central atom is B or Be) 3. Valence shell expansion: Compounds with a central atom with more than eight electrons in its valence shell. (Usually the central atom is in the third, fourth, fifth etc period) 23 2024 6.02 Covalent Bonds and Lewis Structure
Exception: Valence shell expansion Some atoms can accommodate more than an octet especially if the central atom is from the 3rd, 4th, ... period. i.e., PCl6, SF4, AsF6- Modification of Bond Table: Determine the total valence electron. Determine the minimum # of bonds. Determine remaining electrons Re = (TVe-) - (# e- in bonding) Assign remaining electrons to outer most atoms to satisfy octet rule (Except H-atom). Assign all remaining electrons to central atom. 24 2024 6.02 Covalent Bonds and Lewis Structure
Summary Compounds, elements coming together: i) electrons are shared between elements if there is mutually sharing, covalent compounds forms if there is unequal sharing, polar covalent compounds forms. ii) electron transfer occurs, ionic compounds forms (next section). Lewis Structure Determination: i) Molecular Formula ii) Atomic Sequence (H and F are terminal) iii) Determine the # of bonds Oe- and TVe- # of Bonds = (Oe - TVe-) / 2 iv) Determine remaining electrons Re = (TVe-) - (# e- in bonding) v) Make sure all atoms satisfy octet rule (Except H which is satisfied with 2 electrons) 25 2024 6.02 Covalent Bonds and Lewis Structure