Chemical Families on the Periodic Table: Properties and Reactivity
Discover the diverse chemical families on the periodic table and their unique properties that dictate varying reactivities amongst elements. Explore the characteristics of alkali metals, alkaline earth metals, transition metals, boron family, carbon family, and nitrogen family. Gain insights into how elements in each family interact with others and their roles in chemical reactions.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
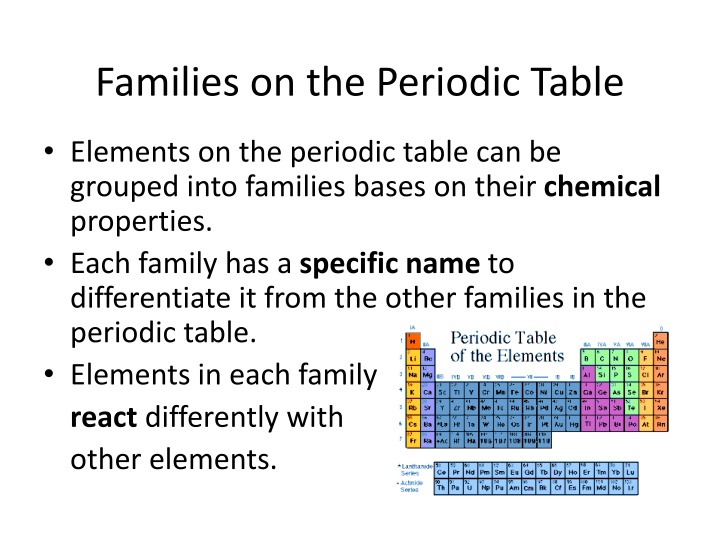
Families on the Periodic Table Elements on the periodic table can be grouped into families bases on their chemical properties. Each family has a specific name to differentiate it from the other families in the periodic table. Elements in each family react differently with other elements.
ALKALI METALS Group 1 Hydrogen is not a member, it is a non-metal C:\Users\Liz\AppData\Local\Microsoft\Windows\Temporary Internet Files\Content.IE5\2B8VNN8W\MCj04260680000[1].wmf All are metals and solid at room temp Soft and silvery, shiny Very reactive, esp. with water Conduct electricity Image: http://www.learner.org/interactives/periodic/groups2.html
ALKALINE EARTH METALS C:\Users\Liz\AppData\Local\Microsoft\Windows\Temporary Internet Files\Content.IE5\2B8VNN8W\MCj04260680000[1].wmf Group 2 Metals Solids at room temp White, silvery Reactive, but less than Alkali metals Conduct electricity
C:\Users\Liz\AppData\Local\Microsoft\Windows\Temporary Internet Files\Content.IE5\2B8VNN8W\MCj04260680000[1].wmf TRANSITION METALS Metals Almost all are solids at room temp (Hg) Good conductors of heat and electricity. Can bond with many elements in a variety of shapes.
BORON FAMILY C:\Users\Liz\AppData\Local\Microsoft\Windows\Temporary Internet Files\Content.IE5\2B8VNN8W\MCj04260680000[1].wmf Group 3 3 electrons in the outer shell Most are metals Boron is a metalloid Reactive Solid at room temp
C:\Users\Liz\AppData\Local\Microsoft\Windows\Temporary Internet Files\Content.IE5\2B8VNN8W\MCj04260680000[1].wmf CARBON FAMILY Group 4 Carbon (C) Reactivity varies Solids at room temp
C:\Users\Liz\AppData\Local\Microsoft\Windows\Temporary Internet Files\Content.IE5\2B8VNN8W\MCj04260680000[1].wmf NITROGEN FAMILY Group 5 5 electrons in the outer shell Can share electrons to form compounds Nitrogen is the only gas at room temp, rest are solids
C:\Users\Liz\AppData\Local\Microsoft\Windows\Temporary Internet Files\Content.IE5\2B8VNN8W\MCj04260680000[1].wmf OXYGEN FAMILY Group 6 6 electrons in the outer shell Reactive Oxygen is a gas, the rest are solids at room temp
C:\Users\Liz\AppData\Local\Microsoft\Windows\Temporary Internet Files\Content.IE5\2B8VNN8W\MCj04260680000[1].wmf Halogens Group 7 7 electrons in the outer shell
C:\Users\Liz\AppData\Local\Microsoft\Windows\Temporary Internet Files\Content.IE5\HJD89WLP\MCj04260680000[1].wmf Noble Gases Group 8 Exist as gases Non-metals 8 electrons in the outer shell = Full Helium (He) has only 2 electrons in the outer shell = Full Not reactive with other elements
C:\Users\Liz\AppData\Local\Microsoft\Windows\Temporary Internet Files\Content.IE5\HJD89WLP\MCj04260680000[1].wmf Rare Earth Metals Some are Radioactive The rare earths are silver, silvery- white, or gray metals. Conduct electricity
Periods Each row is called a period The elements in each period have the same number of shells 1stPeriod = 1 Shell 2ndPeriod = 2 Shells 3rdPeriod = 3 Shells 4thPeriod = 4 Shells www.chem4kids .com
Groups Group 8 = 8 electrons Group 1 = 1 electron Except for He, it has 2 electrons Group 2 = 2 electrons 3, 4, 5, 6, 7 Each column is called a group Each element in a group has the same number of electrons in their outer orbital, also known as shells . The electrons in the outer shell are called valence electrons
Complete Periodic Table of Elements Warm-up worksheet Metals - are on the left side Nonmetals are on the right side Metalloids are on a slanting line between the metals and nonmetals
Complete Periodic Table of Elements Warm-up worksheet Where are the metals, nonmetals, and metalloids located in the periodic table?
Metalloid Elements having properties of both metals and nonmetals.
Complete Periodic Table of Elements Warm-up worksheet Germanium, with an atomic number of 32, is not a metal or a non-metal, but a metalloid. Describe where metalloids are found on the periodic table, with respect to the metals and non-metals.
Complete Periodic Table of Elements Warm-up worksheet Metalloids are found in between metals and non- metals on the periodic table. Their position tells us that they have some of the characteristics of metals and some characteristics of non-metals. For example, germanium conducts electricity, but not as well as true metals.
Elements are organized in the periodic table in such a way that there are patterns of elements placed close together that have similar properties. For example, knowing the properties of one element in a column of the periodic table will help a person predict the properties of other elements in that same column. Describe two properties common to elements found at the far left of the periodic table. Describe two properties common to elements found at the far right of the periodic table.
Complete Periodic Table of Elements Warm-up worksheet On the far left side of the periodic table the elements are all metals. So, they are solids at room temperature and are good conductors of heat and electricity. One the far right side of the periodic table, the elements are gases at room temperature. They are colorless and non- reactive.