Chemical Kinetics: Factors Affecting Reaction Rates and Rate Calculations
Explore the factors influencing reaction rates, reaction mechanisms, and rate calculations in chemical kinetics. Learn about the impact of physical state, reactant concentrations, temperature, and catalysts. Practice exercises and understand the concept of instantaneous rates.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
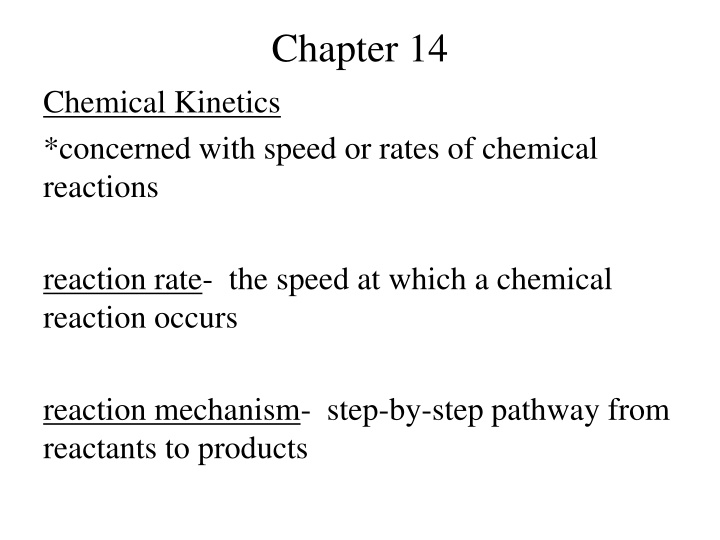
Chapter 14 Chemical Kinetics *concerned with speed or rates of chemical reactions reaction rate- the speed at which a chemical reaction occurs reaction mechanism- step-by-step pathway from reactants to products
Factors That Affect Reaction Rates 1. Physical state of the reactants -if reactants are in different phases, the reaction is limited by the area of contact *inc surface area of solid to inc rate 2. Reactant concentrations *inc conc = inc collisions = inc rate 3. Reaction temp -inc temp = inc KE/collisions = inc rate 4. Presence of a catalyst *inc rate without being used up
rate of appearance of product = [product] time [ ] = concentration rate units = M/s *conc of reactants dec with time while the conc of product inc *rate of disappearance of reactants = rate of appearance of product rate of disappearance = - [reactant] time
Sample Exercise 14.1 page 560 Using data in figure 14.3 pg 559, calculate the rate at which A disappears over the time interval from 20s to 40s. rate of disappearance = - [reactant] time rate = -(0.30M-0.54M) (40s-20s) = 0.012M/s
Practice Exercise rate of product = (0.70M - 0M) (40s - 0s) = 0.018M/s
-it is typical for rates to dec over time WHY? -the conc of reactants dec page 561 Table 14.1 and Fig 14.4
instantaneous rate- rate at a particular instant during a reaction -determined from the slope of the curve at a particular point in time -tangent lines are drawn to find the rate initial rate- instantaneous rate at t=0 Page 561 figure 14.4 *Problems page 562
Rate = - [reactant] = [product] t t aA + bB cC + dD -a,b,c,d are the coefficients from the balanced equation rate = -1 [A] = -1 [B] = +1 [C] = +1 [D] a t b t c t d t
Rate Law -relationship between the rate of the reaction and the conc of the reactants -consider this reaction: aA + bB cC + dD -the rate law would be: rate = k[A]m[B]n k = rate constant m and n = reaction order (small whole numbers) *the value of m and n determines how the rate depends on the conc of the reactant- can only be found experimentally
-to find reaction order, look at two values of a reactant that do not change -see what happens to the rate when the other reactant is changed *if rate change is same as conc change, it is first order (21, 31, 41) ex: conc doubles, rate doubles *if rate change is the square of conc change, it is second order (22, 32, 42) ex: conc doubles, rate quadruples *if rate does not change as the conc changes, it is zero order -do this for each reactant
Example: look at page 563 Table 14.2 rate = k[NH4+]m[NO2-]n *find order of each reactant *m and n are both first order based on data rate = k[NH4+]1[NO2-]1 -solve for k -using experiment #1 data 5.4x10-7M/s = k(0.0100M)1(0.200M)1 k = 2.7x10-4/M s
*can use rate law and k to calculate rate for any set on conc Ex: -find rate of same reaction if each conc is 0.100M rate = 2.7x10-4/M s(0.100M)1(0.100M)1 = 2.7x10-6M/s
-a larger value of k means a fast reaction -a smaller value of k means a slow reaction -units of k change depending on overall order of reaction zero order = Ms-1 1storder = s-1 2ndorder = M-1s-1 -overall order of reaction found by adding together orders of each reactant
First Order Reactions -rate is directly proportional to a single reactant Integrated Rate Law -relationship between intial conc of reactant and conc at any other time ln[A]t = -kt + ln[A]0 y = mx + b *has form of general equation for a straight line *if when t vs. ln[A]tis graphed and a straight line is produced then it is first order
Second-Order Reactions -rate is proportional to the square of the [A] or to reactants each raised to first power -more sensitive to conc of reactants than first order Integrated Rate Law 1 [A]t= kt + [A]0 [A]t= (kt +1/[A]0) *if when t vs. produced then it is second order 1 1 1 [A]tis graphed and a straight line is
Zero-Order Reactions -rate of reaction is independent of the [A] -rate is the same at any concentration Integrated Rate Law [A]t= -kt + [A]0 *if when t vs. [A]tis graphed and a straight line is produced then it is zero order
Half-life -time required for the conc of a reactant to fall to one half of its initial value Zero-Order t1/2 = [A]0 2? First-Order t1/2= 0.693 ? Second-Order 1 t1/2= ?[A]0
Temperature and Rate -as temp inc rate inc Ex- Glow sticks collision model- molecules must collide in order to react -the greater the # of collisions/sec the greater the reaction rate -inc temp and conc of reactants inc the collisions which inc the rate
-for a reaction to occur more is required than just collisions -molecules must be oriented in a certain way to make sure they are positioned to form new bonds -page 576 figure 14.15
-molecules must also possess a certain amount of energy to react -this energy comes from collisions -KE is used to stretch, bend and break bonds activation energy/energy barrier- minimum amount of energy required to initiate a chemical reaction (Ea) -as Eainc reaction rate dec
activated complex/transition state- a high energy intermediate step between reactants and products -page 577 figure 14.17 -for reverse reaction Ea= E + Ea *must change sign on E because going from right -Eawill dec with inc temp -page 578 figure 14.18 -the fraction of molecules that have an energy Ea is given by: f = e-Ea /RT
Arrhenius found that most reaction data obeyed an equation based on: a) the fraction of molecules possessing energy Eaor greater b) # of collisions/sec c) the fraction of collisions that have the appropriate orientation Arrhenius Equation: k = Ae-Ea /RT A= frequency factor related to frequency of collisions and the probability that the collisions are favorably oriented
Sample Exercise 14.10 page 579 Rank slowest to fastest 2 < 3 < 1 Ea= #2= 25kJ/mol #3= 20kJ/mol #1= 15kJ/mol Practice Exercise Reverse from slowest to fastest 2 < 1 < 3 Ea= E + Ea*change sign on E b/c reversing #2= 40kJ/mol #1= 25kJ/mol #3= 15kJ/mol
Reaction Mechanisms -the steps by which a reaction occurs Elementary Reactions -when a reaction occurs in a single event or step molecularity- defined by the # of reactants in an elementary reaction unimolecular- single reactant is involved bimolecular- collision of two reactants termolecular- collision between three molecules (not as probable as uni or bi)
Multistep Mechanisms -sequence of elementary equations ex- page 582 -the elementary reactions in a multistep mechanism must always add to give equation of overall process intermediate- not a reactant or a product, formed in one elementary reaction and consumed in the next
Sample Problem 14.12 page 582 a) Molecularity = uni and bi b) Write equation for overall reaction 2O3(g) 3O2(g) c) Identify intermediates O(g)
Practice Exercise a) yes it is consistent b/c both equations add to give overall reaction b) Molecularity = uni and bi c) Intermediates = Mo(CO)5
Rate Laws for Elementary Reactions -rate law is based on molecularity Table 14.3 page 584 Sample Problem 14.13 page 584
Multistep Mechanisms -each step in a mechanism has its own rate constant and Ea -often one step is much slower than the others -the overall rate cannot exceed the rate of the slowest elementary step rate determining step/rate limiting step- limits overall reaction rate -determines the rate law
Mechanisms with slow initial step Page 585 -since step 1 is slow it is rate-determining step -rate law equals rate law of step 1 rate = k1[NO2]2
Mechanisms with fast initial step Page 587 -step 1 has forward and reverse (k-1) reaction -step 2 is slow and determines overall rate rate = k2[NOBr2][NO] -NOBr2is an intermediate (unstable, low conc.) -rate law depends on unknown conc. -not desirable -want to express rate law in terms of reactants and products