Chemical Kinetics Overview: Reactions, Rate Equations, and Order
Explore the fundamental principles of chemical kinetics, including reaction progress, reaction velocity, and rate equations. Discover the significance of studying reaction mechanisms and the utility of determining the correct mechanism. Learn about reaction rates, stoichiometry, rate equations, and reaction orders in this introductory lecture on chemical kinetics.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Chemical kinetics (Lecture -1)
There are two basic questions for a chemical reaction (i) How far the reaction proceeds? and (ii) How fast does it proceed? # Answer to the question (i) is in the Chemical equilibrium. # Answer to the question (ii) will be given in the chapter of Chemical kinetics.
Depending on velocity chemical reaction can be classified into following three classes: (a) Very fast reaction: very high velocity e.g. explosion reaction; precipitation reaction etc. Generally they are completed within fraction of a second. (b) Very slow reaction: We simply do not measure the rate as our life is not so long e.g. one hundred and fifty years is required for the formation of one drop of water from H2and O2at ordinary temperature without catalyst. (c) Slow reaction: Moderate velocity. We generally study kinetics of reaction of type-(C)
Utility of the Study To know the mechanism of a chemical reactions. Out of a number of proposed mechanism, the correct one can be selected.
Rate of a reaction : Amount of reactant disappearing or amount of product appearing per unit time. Rate with respect to reactant = - Rate with respect to product = # Minus sign is used because the concentration of reactants decreases with time
Reaction involving different stoichiometric Coefficients of reactants and products For a reaction aA + bB = xX + yY rate of reaction = - = = Therefore, rate of disappearance of A,
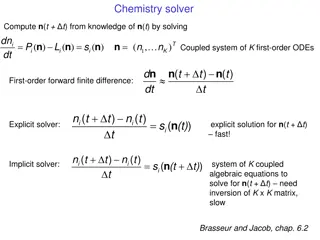
Rate equation and rate constant The general form of rate equation for an reaction is = KCn - Here C means concentration of reactant at the time of measuring rate. n is called order and K is rate constant or specific reaction rate
Order of a reaction: Reactions and their rate equation as expected from law of mass action. REACTION 1. A product 2. A+B Product RATE OF EQUATION Rate CA Rate CA, CB Reaction (1) is 1storder and reaction (2) is second order. However, 2HI = H2+ I2 may appear as second order but in Au- surface at high pressure, rate of the reaction is zero. So, order is the sum of concentration terms on which the rate of the reaction actually depends.
Molecularity of a reaction: # Molecularity of a reaction is the number of molecules participating in each elementary step of a reaction. # Some times molecularity of the slowest step (rate determining step) is taken as overall molecularity of the reaction. # Knowledge of molecularity requires a knowledge of all intermediate steps i.e. mechanism.
ORDER MOLECULARITY 1. Order is number of conc. terms appearing in rate equation 1. Molecularity is number of molecules taking part in each step of reaction. 2. Order is an experimentally determinable quantity. 2. Molecularity is a theoretical quantity Order may be fraction, -ve; zero or whole number. 3. 3. Molecularity is always a whole number. 4. Knowledge of order does not require knowledge of mechanism. 4. Knowledge of molecularity requires a knowledge of mechanism. 5. Order depends on external factors e.g. P,T governing the reaction. 5. Molecularity does not depend on such factors.
FIRST ORDER KINETICS A first order reaction is a reaction, whose rate determining step consists of only one reactant molecule e.g. A Product If is the starting concentration of A and C is its concentration after time t then rate ( dc/dt ) at this time will be = rate constant or
Problems: 1.Show that for a first order reaction time required for 75% reaction is twice the time for 50% reaction. 2. The rate of decomposition of gas was 7.25 in some unit when 5% had reacted and it was 5.14 in the same unit when 20% had gone decomposition. Calculate the order.