Chemical Properties of Substances: Sodium Chloride, Calcium Chloride, Ammonium Chloride, Potassium Chloride, Hydrogen Chloride
Sodium chloride, calcium chloride, ammonium chloride, potassium chloride, and hydrogen chloride are discussed in terms of their physical properties, common uses, and reactions with water. These substances have unique characteristics, such as melting points, boiling points, and densities, that define their behavior and applications in various industries. By understanding the chemical properties of these substances, one can appreciate their significance in everyday life and specialized fields like medicine, agriculture, and manufacturing.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Chemical Properties of Substances Made By: Feras Abu Jiries, Sanad Made By: Feras Abu Jiries, Sanad Salhi and Zaid Bakeer Salhi and Zaid Bakeer
Sodium Chloride Sodium chloride is a white crystalline solid that contains a density of 2.165 g/mL . It has a melting point of 801 C and a boiling point of around 1,413 C and it is commonly known as table salt and it is used as a seasoning for food. When Sodium chloride is added to water it absorbs heat from it s surroundings (water) which makes it an endothermic reaction.
Calcium Chloride Calcium chloride is a white silvery cubic crystal that contains a density of 1.55 g/cm3 at 293 K. It has a melting point of 772 C and a boiling point of 1965 C and is commonly used for the treatment of hypocalcemia and hyperkalemia. When Calcium chloride is added to water it gives out heat to it s surroundings (water) which make it an exothermic reaction.
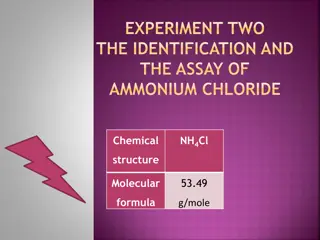
Ammonium Chloride Ammonium chloride is awhite crystalline solid that has no smell that contains a density of approximately 1.5274 g mL-1. It has a melting point of 338 C and a boiling point of 520 C and is commonly used as a nitrogen supply in fertilizers and as an electrolyte in dry cells. When Ammonium chloride is added to water it absorbs heat from it s surroundings (water) which makes it an endothermic reaction.
Potassium Chloride Potassium chloride is awhite crystalline solid that contains a density of 1.98g/cm . It has a melting point of 770 C and a boiling point of 1,420 C and it is commonly used to prevent or treat low potassium levels in the body. When Potassium chloride is added to water it absorbs heat from it s surroundings (water) which makes it an endothermic reaction.
Hydrogen Chloride Hydrogen chloride is a colorless gas that contains a density of 1.49kg/m . It has a melting point of -114.2 C and a boiling point of -85.05 C and it is commonly used for cleaning, pickling, electroplating metals, tanning leather, and refining. When Hydrogen chloride is added to water it gives out heat to it s surroundings (water) which make it an exothermic reaction.