Chemical Reaction Rate Laws and Equilibrium Review
Explore reaction rates, rate laws, equilibrium constants, and conversion processes in chemical reactions. Understand how temperature and concentration affect reaction rates and equilibrium. Learn key concepts like Arrhenius equation, stoichiometry, and determining reaction order.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
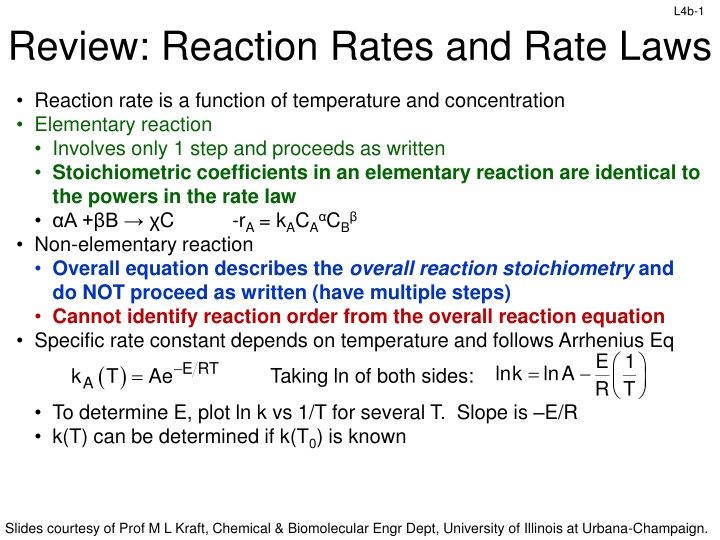
L4b-1 Review: Reaction Rates and Rate Laws Reaction rate is a function of temperature and concentration Elementary reaction Involves only 1 step and proceeds as written Stoichiometric coefficients in an elementary reaction are identical to the powers in the rate law A + B C -rA = kACA CB Non-elementary reaction Overall equation describes the overall reaction stoichiometry and do NOT proceed as written (have multiple steps) Cannot identify reaction order from the overall reaction equation Specific rate constant depends on temperature and follows Arrhenius Eq ( ) A k T = Taking ln of both sides: E 1 R T Ae E RT = lnk lnA To determine E, plot ln k vs 1/T for several T. Slope is E/R k(T) can be determined if k(T0) is known Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois at Urbana-Champaign.
L4b-2 Review: Reversible Reactions kA kA + + aA b B c C d D a b c d = = + = + A,net r r forward,A r backwards,A r r k C C k C C A A A A B A C D At equilibrium, the reaction rate is zero, rA=0 c d Thermodynamic equilibrium relationship C C C C k k C D A = = K C a b KC: concentration equilibrium constant (capital K) A A B H 1 T 1 T KC is temperature dependent (no change in moles or CP): RX R = K (T) K (T )exp C C 1 1 HRX: heat of reaction If KC is known for temperature T1, KC for temperature T can be calculated Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois at Urbana-Champaign.
Review: Conversion is Everything! Batch: We need to determine how long to leave the reactants in the reactor to achieve a certain conversion X X A dX A V = t N 0 A 0 r A CSTR: -1/rA We need to determine the reactor size (volume) required to achieve a certain conversion X F A0 r = V X A A X PFR: -1/rA We need to determine the reactor size (volume) required to achieve a certain conversion X X A dX A = V F 0 A 0 r A X But rA depends on Cj, and Cj is a function of XA We need to relate rA to XA Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois at Urbana-Champaign.
L4b-4 Review: Derive rA = f(XA) j stoichiometric coefficient for products, for reactants j r j = r A Relate all rj to Cj Batch: Flow: ( ) ( ) + + j A0 F N V N j A0 N V X F F X j j0 A j j0 A = = = = C C Relate all Cj(XA) to V( ) j j Batch: Flow: P P P P T T Z Z Z Z T T ( ) ( ) 0 0 = + = + V V 1 X 1 X Relate all V( ) to XA 0 A 0 A 0 0 0 0 Batch & Flow: N N + + C C X T T0 T T Z Z P P = j0 A0 X j A 0 0 = C N Put j T0 1 A 0 together Now that Cj is in terms of XA, we can write the rate law in terms of XA Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois at Urbana-Champaign.
Chapter 3 Examples Rate Laws and Stoichiometry Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois at Urbana-Champaign.
L4b-6 The following reaction is irreversible at low temperature, but reversible at high temperature. kf and KC are known, but kbisn t. Suggest a rate law for the high temperature reaction. A B = r kC A A Reversible reaction: A B = Forward rxn rate: r k C A f A = Reverse rxn rate: r k C A b B Need kb in known terms = r k C b B k C Put it together to get rA: A f A At equilibrium, -rA = ? a) -rA >0 b) -rA<0 c) -rA=0 d) -rA=1 e) None of the above Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois at Urbana-Champaign.
L4b-7 The following reaction is irreversible at low temperature, but reversible at high temperature. kf and KC are known, but kbisn t. Suggest a rate law for the high temperature reaction. A B = r kC A A Reversible reaction: A B = Forward rxn rate: r k C A f A = Reverse rxn rate: r k C A b B Need kb in known terms = r k C b B k C Put it together to get rA: A f A = = At equilibrium, -rA = 0 r 0 k C b B k C Solve for kb A f A products C C k C C C f C A = = B b B k C k C k = = K We know that: f A b C B reactants A k C k K f C A f = = so k k Substitute this expression for kb into -rA b b B C k K f = = r k C b B k C r k C C A f A A f A B C Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois at Urbana-Champaign.
L4b-8 3 2 ( ) g ( ) g ( ) ( ) + + CH O HCOOH g H O g 4 2 2 What is the relationship between rCH4 and rO2? Use CH4 for basis r j = r j = CH4 O2 A = j = A j What is j? a) 0.5 b) -0.5 c) 3/2 d) -3/2 e) None of the above Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois at Urbana-Champaign.
L4b-9 3 2 ( ) g ( ) g ( ) ( ) + + CH O HCOOH g H O g 4 2 2 What is the relationship between rCH4 and rO2? Use CH4 for basis r j = r j = CH4 O2 -3/2 A = j = A j Negative because O2 is a reactant r O2 3 2 )( ) = r ( = = 3 2 CH r O r 1.5r O r Plug in: CH4 CH 4 2 4 2 O2 is consumed 1.5x faster than CH4 Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois at Urbana-Champaign.
L4b-10 N2(g) + 3/2 H2(g) NH3(g) The formation of ammonia is to be carried out isothermally at 227 C and with a pressure of 16.4 atm. This is an isobaric (constant pressure) flow system with equimolar feeds of N2 & H2. Assume the gas mixture behaves like an ideal gas. Clicker Question 2: Does the volumetric flow rate, , change as a function of conversion, XA? a) Yes b) No c) Not enough information to tell 1 P P Z Z T T ( ) 0 = + 1 X 0 A 1 0 0 1 change in total # moles at XA=1 N N T T0 = N T0 total moles fed 0, so changes as a function of conversion Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois at Urbana-Champaign.
L4b-11 N2(g) + 3/2 H2(g) NH3(g) The formation of ammonia is to be carried out isothermally at 227 C and with a pressure of 16.4 atm. This is an isobaric (constant pressure) flow system with equimolar feeds of N2 & H2. Assume the gas mixture behaves like an ideal gas. Taking H2 as your basis, fill in the stoichiometric table for the rxn above Normalize in terms of H2 by multiplying the equation by : H2 + N2 NH3 Compound H2 N2 NH3 Total Symbol A B C In Change Out FB0=FA0 0 2FA0 The feed contains equimolar amounts of N2 and H2 FA0 = FB0 The feed does not contain product Add it up FA0 FB0 Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois at Urbana-Champaign.
L4b-12 N2(g) + 3/2 H2(g) NH3(g) The formation of ammonia is to be carried out isothermally at 227 C and with a pressure of 16.4 atm. This is an isobaric (constant pressure) flow system with equimolar feeds of N2 & H2. Assume the gas mixture behaves like an ideal gas. Taking H2 as your basis, fill in the stoichiometric table for the rxn above Normalize in terms of H2 by multiplying the equation by : H2 + N2 NH3 Compound H2 N2 NH3 Total Symbol A B C In FA0 Change Out FB0=FA0 0 2FA0 = How much A is consumed in mol/time? F F F X Change in H2: A A 0 A 0 A Molar flow rate A is fed to reactor Molar rate A is consumed in reactor Molar flow rate A leaves reactor - = Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois at Urbana-Champaign.
L4b-13 N2(g) + 3/2 H2(g) NH3(g) The formation of ammonia is to be carried out isothermally at 227 C and with a pressure of 16.4 atm. This is an isobaric (constant pressure) flow system with equimolar feeds of N2 & H2. Assume the gas mixture behaves like an ideal gas. Taking H2 as your basis, fill in the stoichiometric table for the rxn above Normalize in terms of H2 by multipyling the equation by : H2 + N2 NH3 Compound H2 N2 NH3 Total Symbol A B C In FA0 Change -FA0XA Out FB0=FA0 0 2FA0 - FA0XA = F F F X Change in H2: A A 0 A 0 A + ( ) )A0 ) A0 )A0 ( = + j A0 F F F X = 1 3 F 1 3 F X X F B0 F Change in N2: j j0 A A A B A0 +( ( Change in NH3: Add up the total change = + j A0 F = F F X F C0 F 2 3 F 2 3 F X X j j0 A C A A Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois at Urbana-Champaign.
L4b-14 N2(g) + 3/2 H2(g) NH3(g) The formation of ammonia is to be carried out isothermally at 227 C and with a pressure of 16.4 atm. This is an isobaric (constant pressure) flow system with equimolar feeds of N2 & H2. Assume the gas mixture behaves like an ideal gas. Taking H2 as your basis, fill in the stoichiometric table for the rxn above Normalize in terms of H2 by multipyling the equation by : H2 + N2 NH3 FB0=FA0 Compound H2 N2 NH3 Total Symbol A B C In FA0 Change -FA0XA -( FA0XA) FA0XA - FA0XA Out FA0-FA0XA FA0-( FA0XA) FA0XA FA0(2- XA) FB0=FA0 0 2FA0 = F F F X H2 Out H2: A A 0 A 0 A ( ) = + j A0 F F F X N2 Out = + F B0 F 1 3 F X N2: j j0 A B A0 A ( ) NH3: = + j A0 F = + F F X F C0 F 2 3 F 0 X NH3 Out j j0 A C A0 A Add up the total change Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois at Urbana-Champaign.
H2 + N2 NH3 L4b-15 The formation of ammonia is to be carried out isothermally at 227 C and with a pressure of 16.4 atm. This is an isobaric (constant pressure) flow system with equimolar feeds of N2 & H2. Assume the gas mixture behaves like an ideal gas. Compound Symbol In H2 A FA0 N2 B FB0=FA0 NH3 C 0 Total 2FA0 What are and CA0? change in total # moles 1 Moles A reacted Change -FA0XA -( FA0XA) FA0XA - FA0XA Out FA0-FA0XA FA0-( FA0XA) FA0XA FA0(2- XA) 2 3 1 3 = + = 1 1 c b c= B=- = = + c b = - change in total # moles at XA=1 N N N N T T0 A0 = = y where y = =mole fraction of A initially present A0 A0 N T0 T0 total moles fed 2 3 1 2 1 3 x 1 equimolar feeds of N2 & H2 = = = = y y = = A0 A0 2x 2 Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois at Urbana-Champaign.
H2 + N2 NH3 L4b-16 The formation of ammonia is to be carried out isothermally at 227 C and with a pressure of 16.4 atm. This is an isobaric (constant pressure) flow system with equimolar feeds of N2 & H2. Assume the gas mixture behaves like an ideal gas. Compound Symbol In H2 A FA0 N2 B FB0=FA0 NH3 C 0 Total 2FA0 What are and CA0? = - A0 y N F mol C V volume Do have P & T N P C RT V Change -FA0XA -( FA0XA) FA0XA - FA0XA 1 3 Out FA0-FA0XA FA0-( FA0XA) FA0XA FA0(2- XA) 1 2 = = Don t have FA0 or 0 A0 A0 = = = A0 Relate P&T to CA0: 0 T0 = = = This is CT0! How is NA0 related to NT0? PV N RT T0 T0 N 0.5N 1 2 0.5P RT mol L A0 V T0 = = = = N N C N2 & H2 are equimolar in the feed: A0 T0 A0 V We divided by P 2 instead of multiplying it by 0.5 16.4atm atm L mol K = = C C 0.2 P= 16.4 atm; T= 227 C=500K A0 A0 2 0.082 500K Plug in: Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois at Urbana-Champaign.
H2 + N2 NH3 L4b-17 The formation of ammonia is to be carried out isothermally at 227 C and with a pressure of 16.4 atm. This is an isobaric (constant pressure) flow system with equimolar feeds of N2 & H2. Assume the gas mixture behaves like an ideal gas. Compound Symbol In H2 A FA0 N2 B FB0=FA0 NH3 C 0 Total 2FA0 What are the concentrations of H2, N2, and NH3 when H2 is 60% consumed? + = + A 0 1 X P T Z 1 1 1 1 3 L ( )( )( ) ( ) 1 13 0. 6 L + Change -FA0XA -( FA0XA) FA0XA - FA0XA Out FA0-FA0XA FA0-( FA0XA) FA0XA FA0(2- XA) isothermal isobaric C C X T Z P + 1 C C X j0 j A0 A 0 0 j0 A0 X j A C = C j j + A ideal ( 13 0.6 )( )( 13 0.6 )( ) 0.2 1 0.2 0.6 1 + ( ( + mol mol L = = = = X 0.6 = = CC0=0 C 0.2 C C 0.1 A A0 B0 H2 ( )( )( ) ) + + 0.2 13 0.2 0.6 0 2 3 0.2 0.6 mol mol L = = = = C 0.2 C 0.1 N2 NH3 ) 1 Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois at Urbana-Champaign.
L4b-18 A reversible reaction is carried out in a CSTR. What is the CSTR volume when XA=0.9, CA0=2 mol/m3, FA0=2 mol/min, and there is no product in the feed stream? ( ) ( ) k2 A + B Check if XA=0.9 is obtainable by determining XA when the rxn is at equilibrium (-rAe=0): 2 Ae 1 Ae 2 Be r 0 k C k C = = C = Put CAe and CBe in terms of XAe: ( ) Be B0 A0 Ae C C C X ; C = + A = k 1L mol min 1 min = 1 k1 2 = r 1 A k C 2 B k C A l B l A 1 k 2 2 Rearrange = 2 Be k C X 1 Ae k C C ( A0 X ( 1 A0 k C ( ) ( ) = 1 X C C C ) Ae A0 A0 Ae Ae A0 Ae = = 0 ( C ) C B0 ( C Be ) Ae )2 2 = 1 X k X Plug CAe and CBe into rate eq: 2 A0 Ae Ae 2 k 1 1 2X + X k 2 )2 ( Ae X Ae 2 + = + 2 X = = 1 X k X 1 A0 k C Ae 2 Ae Ae 1 A0 k C X 1 A0 k C Ae Ae 1min 1 Plug in values of variables & simplify + = + 2 X ( )( ) Ae 3 3 X 1m mol min 2mol m X Impossible Ae 1 2 1 + = + 2 X XAe =2 or XAe=0.5 0.5 =XAe< XA=0.9 Cannot obtain XA of 0.9! Ae X Ae Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois at Urbana-Champaign.