Chemical Reactions and Balancing Equations Guide
Explore the world of chemical reactions and learn how to balance equations with this comprehensive guide, covering evidence of chemical reactions, chemical equation notation, balancing techniques, and helpful tips and examples. Enhance your understanding of balancing chemical equations and master the art of representing chemical reactions symbolically. Dive into the fascinating realm of chemistry and uncover the secrets behind the laws of conservation of mass. Get ready to excel in your chemistry studies with this informative resource!
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Chemistry Review Objective Agenda Balancing Reactions ID chemical reactions Chemical equation notation Balance equations Write equations from word descriptions Assignment: Balancing Reactions Worksheet (Skip instructions for Blank on left on p2. Just balance for now.)
Evidence of a possible chemical reaction (review) Color change (ex: rust formation) Formation of a solid (ex: hard water deposits) Formation of a gas (ex: Alka Seltzer) Emission of light (ex: fireworks) Spontaneous exchange of heat (heats up or cools down) (ex: hot/cold packs) These observations usually indicate a chemical change Phase changes also involve the formation of a solid or a gas, usually only due to heat addition/subtraction, not mixing another substance
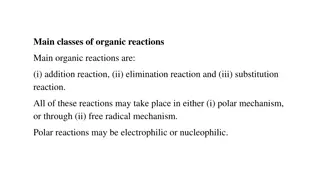
Chemical Equations Chemical reactions are represented symbolically using chemical equations Ex: 2 C2H6 (g) + 7 O2(g) 4 CO2(g) + 6 H2O (g) The substances mixed together are called reactants. Their chemical formulas are listed with a + sign between them. Then an arrow represents the process of reacting. Any additional information about the reaction process may be written above or below the arrow. (Heat, light, catalyst ) The substances produced from the reaction are called products. Their formulas are listed with a + sign between them. Often, the state of each substance is stated in parentheses. (s, l, g, aq) optional Large numbers in front of each formula are called coefficients and are necessary to balance the equation.
Balancing Chemical Equations Because of the law of conservation of mass, the same number and types of atoms must be present on both sides of a chemical equation. Atoms are neither created nor destroyed in a chemical reaction but only reorganized. Balance an equation by adding coefficients in front of chemical formulas as needed. DO NOT ADD OR CHANGE FORMULA SUBSCRIPTS! (Verify all formulas are correct before balancing.)
Balancing Chemical Equations How to balance: 1. Make an inventory of the number and type of each type of element on each side of the equation. 2. Balance one element. Update the inventory. 3. Repeat for each element until all elements are balanced. 4. Verify each type of atom is balanced.
Balancing equations Examples _____Li2O + _____H2O _____LiOH 1 1 2 Li 2 2 1 2 2 1 O 2 H 1 2
Some strategies and hints Avoid balancing an element that occurs in multiple compounds on one side until later. These elements often balance when other elements balance. Balance a free element last. When there is not a simple multiple of the numbers, try swapping numbers. When balancing a last diatomic element that needs an odd number of atom, double all other coefficients and then balance the diatomic.
Balancing equations Examples 6 6 5 _____NH3 + _____NO 10 _____ N2 + _____H2O N 2 2 6 12 4 2 3 3 5 3 2 3 10 12 6 6 H 3 O 3 6 1 1
Balancing equations Examples 1 1 3 _____FeCl3 + _____NH4OH _____Fe(OH)3 +_____NH4Cl Fe3+ Cl- NH4+ OH- 3 3 1 1 3 1 3 1 1 3 3 1 3
Balancing equations Examples _____C5H12 + _____O2 _____CO2 + _____H2O 1 8 5 6 As you practice balancing you ll be able to do it without the inventories, but if you get stuck, the inventory will always work.
Word Equations Hint: 9 molecular elements: H2, N2, O2, F2, Cl2, Br2, I2, P4, S8 When solid sodium metal reacts with liquid water, an aqueous solution of sodium hydroxide and hydrogen gas are formed. H2O(l) Na(s) + NaOH(aq) + H2(g) 2 2 2 1
Word Equations Hint: 9 molecular elements: H2, N2, O2, F2, Cl2, Br2, I2, P4, S8 When aqueous calcium chloride and aqueous sodium sulfate are mixed a white solid of calcium sulfate forms in an aqueous solution of sodium chloride. Na2SO4(aq) CaCl2(aq) + CaSO4(s) + NaCl(aq) 1 1 2 1
Word Equations Hint: 9 molecular elements: H2, N2, O2, F2, Cl2, Br2, I2, P4, S8 The reaction of aqueous silver nitrate and aqueous sodium chromate that forms solid silver chromate and aqueous sodium nitrate. AgNO3(aq) + NaNO3(aq) Na2CrO4(aq) Ag2CrO4(aq) + 1 1 2 2
Assignment What s Due? (Pending assignments to complete.) Complete the Balancing Reactions Worksheet Ignore the instructions on the top of pg2 and leave the blanks on the left for our next lesson.