Chemical Reactions and Predictions
Explore various chemical reactions such as combustion and synthesis, learn to predict products, and understand the types of reactions through practical examples and diagrams.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
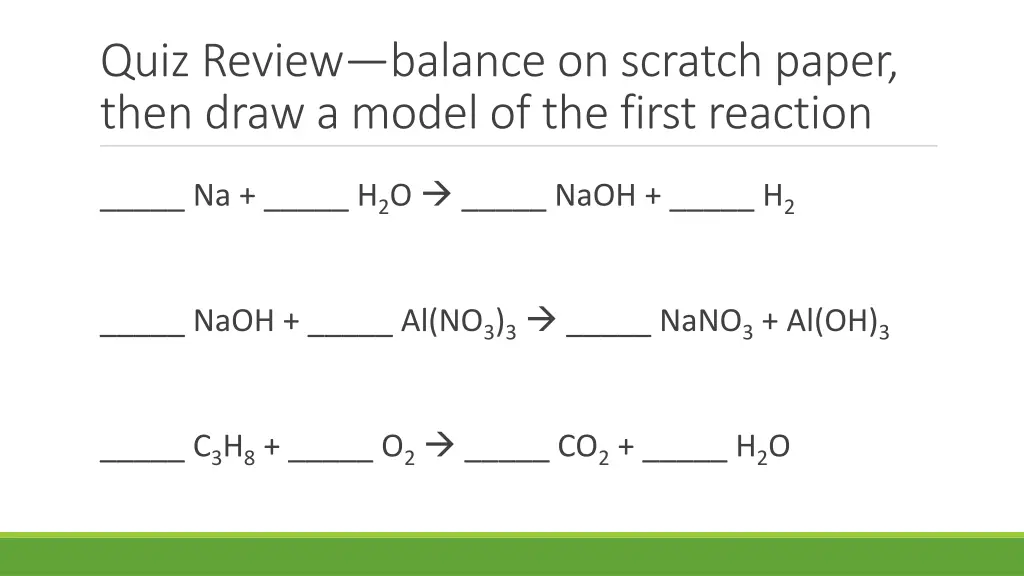
Quiz Reviewbalance on scratch paper, then draw a model of the first reaction _____ Na + _____ H2O _____ NaOH + _____ H2 _____ NaOH + _____ Al(NO3)3 _____ NaNO3+ Al(OH)3 _____ C3H8+ _____ O2 _____ CO2+ _____ H2O
What type of reaction? NaOH + CuSO4 Na2SO4+ Cu(OH)2 C4H12+ O2 H2O + CO2 Fe + O2 Fe2O3 Mg3(PO4)2 + H2 Mg + H3PO4 NH4NO3 N2O + H2O
What type of reaction will happen? C6H14 + O2 Al + F2 CaCO3
Predicting Products for Combustion Rxns Reactants are always a hydrocarbon and O2 Products are always CO2 and H2O Write the formula, then balance!
Predicting Products for Combustion Rxns O2 C6H14 +
Practice O2 C3H8+
Predicting Products for Synthesis Rxns Reactants are always single elements Combine to make an ionic compound - use charges to determine the compound Then balance!
Diatomic Elements Some atoms are diatomic they come in pairs when they are single elements Does NOT apply to ionic compounds only when by themselves!
Common mistake: H2O Why is there only one oxygen in H2O? I thought oxygen was diatomic and could only come in pairs? Shouldn t it be H2O2?
Practice Mg + N2