Chemical Transport Reactions and Concepts
Explore the principles and kinetics of chemical transport involving nonvolatile species moving along temperature gradients. Learn about the processes, equilibrium, and thermodynamics of transport reactions within containers. Discover the factors influencing the direction and rate of chemical transport as explained by diffusion and molecular flow theory.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
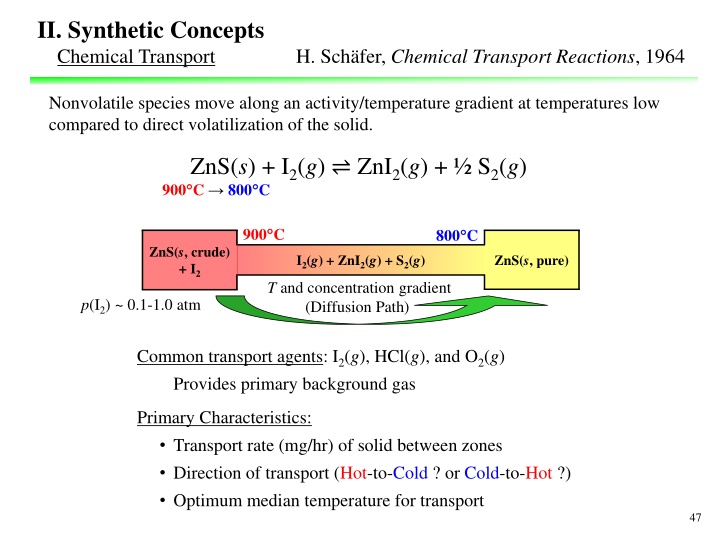
II. Synthetic Concepts Chemical Transport H. Sch fer, Chemical Transport Reactions, 1964 Nonvolatile species move along an activity/temperature gradient at temperatures low compared to direct volatilization of the solid. ZnS(s) + I2(g) ZnI2(g) + S2(g) 900 C 800 C 900 C 800 C ZnS(s, crude) + I2 ZnS(s, pure) I2(g) + ZnI2(g) + S2(g) T and concentration gradient (Diffusion Path) p(I2) ~ 0.1-1.0 atm Common transport agents: I2(g), HCl(g), and O2(g) Provides primary background gas Primary Characteristics: Transport rate (mg/hr) of solid between zones Direction of transport (Hot-to-Cold ? or Cold-to-Hot ?) Optimum median temperature for transport 47
II. Synthetic Concepts Chemical Transport: Principles Process (1) Heterogeneous reaction of B(g) on A(s) (2) Motion of B(g) + C(g) in the container (3) Heterogeneous reaction to reform A(s) Transport Equilibrium iA(s) + jB(g) kC(g) Solid to be Transported Agent Usually rate- determining Transport Gaseous Species (pB + pC) Nature of the gas motion depends on total gas pressurein the (closed) container: (a) Low total pressure (< 10 3 atm) Mean free path of gas molecules container dimensions Tendency to equalize pressure throughout the container (b) Intermediate total pressure (> 10 3 atm) Uniform gas density (constant pTOT) but composition gradient Tendency to equalize concentration throughout the container Diffusion rate decreases as pTOT increases (c) High total pressure Collision frequency of gas molecules is high Tendency to equalize temperature throughout the container Molecular Flow Diffusion Convection 48
II. Synthetic Concepts Chemical Transport: Kinetics Transport Rate pB(T2) pB(T1) nA moles; time t ? iA(s) + jB(g) kC(g) A(s, crude) + B A(s, pure) B(g) + C(g) T2 T1 What is the rate of chemical transport ? (# moles A(s) transported nA during time t) Use reaction stoichiometry and diffusion/molecular flow theory: 0.8? ? ?? ? ? ? ?? ?TOT ?2 Transport 1.8 10 4 moles/hr Rate Chemical Controls Maximize pB Physical Controls Keep tubes as wide and short as possible Maximize high temperature ?? ?TOT is desirable > 10 3 Minimize pTOT (amount of transport agent) pB = pB(T2) pB(T1) pTOT = pB + pC A = cross sect. area of reaction tube (cm2) s = tube length (cm) 49 H. Sch fer et al., Z. anorg. Allg. Chem. 286, 27-55 (1956)
II. Synthetic Concepts Chemical Transport: Thermodynamics Transport Direction T2 > T1-or- T2 < T1 ??? H = ? iA(s) + jB(g) kC(g) A(s, crude) + B A(s, pure) B(g) + C(g) T2 T1 What is the direction of chemical transport ? Hot-to-Cold OR Cold-to-Hot H > 0 Endothermic: High T to Low T (Hot-to-Cold) Favored at Low T Favored at High T iA(s) + jB(g) kC(g) increases As T increases, K __________ . H < 0 Exothermic: Low T to High T (Cold-to-Hot) Favored at High T Favored at Low T iA(s) + jB(g) kC(g) decreases As T increases, K __________ . 50 H. Sch fer et al., Z. anorg. Allg. Chem. 286, 27-55 (1956)
II. Synthetic Concepts Chemical Transport: Thermodynamics Optimum Temperature(s) Exothermic Endothermic ? = 0 J/mol K 1000 pB (atm) ??? =?? =1 ?? A(s) + B(g) C(g) ?? ?? pB(T2) > pB(T1) A(s) forms at T2 pB(T2) < pB(T1) A(s) forms at T1 pTOT = pB + pC = 1 atm T1 = 1073 K, T2 = 1273 K ? ?? ??? = ? ? ??? ? ? ??? = ? H0 (kJ/mol) 1 Plot ?? vs. ? for fixed ? 1000 pB (atm) ??? = 1 + ??? pB(T2) < pB(T1) A(s) forms at T1 Only endothermic equilibrium allows transport ? = +50 J/mol K ???2 ???1 A reaction with large | H0| gives no measurable transport; I2: M I bonds are weak; small | H0| For large | S0|, transport is only possible when H0 and S0 have the same sign; If S0 is small, then sign of H0 controls transport direction; Transport is significant when ln Kp ~ 0 or G ~ 0. H0 (kJ) ? = 10 J/mol K 1000 pB (atm) pB(T2) > pB(T1) A(s) forms at T2 Exothermic equilibrium optimizes transport Optimum median temperature for transport: ? ~ 0: ?med ~ ? ? 51 H0 (kJ/mol)
II. Synthetic Concepts Chemical Transport: Example Consider growing Nb3Cl8(s) crystals by chemical transport in a silica tube (20 cm long, 1 cm diameter). Crude Nb3Cl8(s) is prepared by reacting Nb(s) with NbCl5(s). For transport, place crude Nb3Cl8(s)and NbCl5(s) in one end. NbCl5(g)becomes the transport agent, loaded to give 1 atm total pressure, and NbCl4(g) is another gas-phase component. (a) What is the transport equilibrium? Nb3Cl8(s) + 4 NbCl5(g) 7 NbCl4(g) 1 cm diameter; A = 0.785 cm2 Need ~0.065g NbCl5 for 1 atm pressure (b) What is the direction of transport? H0 = + 457.3 kJ/mol Nb3Cl8; S0 = + 487.9 J/K mol Nb3Cl8; Endothermic equilibrium: transport high-to-lowT; place reactants in hot end. (c) What is the optimum median temperature for transport? ? ? = 457.3 kJ/mol 0.4879 kJ/mol K = 937 K = 664 C G0 ~ 0 at (d) Estimate the transport rate (in moles Nb3Cl8(s) / hour) if ??????~ 0.25 atm. 937 K0.80.785 cm2 20 cm 20 cm 20 cm 20 cm 937 K0.80.785 cm2 937 K0.80.785 cm2 937 K0.80.785 cm2 ??????? ? ? ? ??????? ??????? ??????? ? ??????? ? 1 4 4 4 4 1 1 1 0.25 atm 1 atm 1 atm 1 atm 1 atm 0.25 atm 0.25 atm 0.25 atm = 1.8 10 4 = 1.8 10 4 = 1.8 10 4 = 1.8 10 4 = 1.05 10 4 mol/hr = 1.05 10 4 mol/hr = 1.05 10 4 mol/hr = 1.05 10 4 mol/hr Can grow 1 gm in 17 hours = 0.059 g/hr 52
II. Synthetic Concepts Chemical Transport: Example H > 0 T > 875 C Nb3Cl8(s) 4 Nb(s) + 8 NbCl5(g) Nb3Cl8(s) + 4 NbCl5(g) 7 NbCl4(g) T < 850 K Another competing phase is Nb3Cl7(s) = Nb6Cl14(s) 7 ??? =?????? 7??? ?????? 4 ??????= ??????? ? = 9.28 10 5mol 4 ?????? = 0.052 g/hr hr ??????= ?TOT ?????? T = 750-850 K pi~ 0.25 atm Use Kp(T) and pTOT ~ 1 atm to determine pgas: Optimal Range Decomposition 1.0 T (K) ?? ?????? ?????? ???? NbCl4(g) 0.8 4.7 10 15 600 0.995 0.009 1.004 2.3 10 9 700 0.945 0.056 1.001 0.6 pi (atm) Nb3Cl8(s) grows here 4.2 10 5 800 0.795 0.208 1.003 0.4 8.8 10 2 900 0.520 0.486 1.006 0.2 NbCl5(g) 4.0 101 1000 0.245 0.757 1.002 0.0 600 700 800 900 1000 (atm) T (K) 53
II. Synthetic Concepts Chemical Transport: Reactions 1000 800 C 2 mg I2 / cm3 gives complete conversion ??? ? + ??? ????? +1 2??? 800 C Low conversion due to protective skin that grows on the metal surface and prevents further reaction Zn ? + S ? ??? ? 80 200 C Efficiently purifies noble metals ?? ? + 4 ?? ? ?? ???? H0 < 0: Transport cold-to-hot ? ? + 3 ???? ????? Sustain W filaments in an atmosphere with a small partial pressure of WCl6(g) W in thicker (colder) parts of filament transports to thinner (hotter) parts of the filament Very slow M ? + ? P ?,red ???? 2 ? ?,red + 2 ??? ????? M ? +? 2 ????? ???? + 2? ??? A small amount of I2 transports red P by forming P2I4(g), which then forms metal phosphides and regenerates the transport agent. M. Lenz, R. Gruehn, Chem. Rev. 1997, 97, 2967-2994 R. Gruehn, R. Glaum, Angew. Chem. Int. Ed. Engl. 2000, 39 692-716 P. Schmidt, et al., Chemical Vapor Transport Reactions Methods, Materials, Modeling: https://www.intechopen.com/chapters/43029 54
II. Synthetic Concepts Chemical Transport: Reactions Synproportionation Reactions: Typically S0 > 0 and Endothermic ?? ? +1 2?????? 3 2???? ? 1000 600 C (m.p.(Al) = 660 C) ?? ? + ?????? 2 ?????? 1100 900 C (m.p.(Si) = 1410 C) Separation / Purification Reactions: mixture of M(s) and M (s) (1) M(s) transports but M (s) does not: Nb(s) and NbC(s) with I2 Exothermic: cold-to-hot transport Load Nb(s)/NbC(s) with I2(s) in cold end ?? ? + 2 ??? ????? (2) M(s) transports by exothermic equilibrium; M (s) by endothermic equilibrium: Cu(s) and Cu2O(s) with HCl(g) 3 ?? ? + 3 ??? ? ??????? +3 H = +19 kJ/mol 900 600 C 2 ??? H = 92 kJ/mol 600 900 C 3 2???? ? + 3 ??? ? ??????? +3 2??? ? Cu Cu2O 900 C 600 C Cu2O/Cu Mixture 55
II. Synthetic Concepts Summary Fundamental Concepts Stability: Relative concept; thermodynamic vs. kinetic aspects Diffusion and Nucleation: achieving homogeneous crystalline products High Temperatures: Furnaces, Containers Strategies Crystal Growth Techniques: Solid-phase; Gas-phase; Liquid-phase; Solution-phase Examples: High pressure; Eutectic solvents or fluxes; Vapor transport; ... Quality Control: Planning and analysis Phase Diagrams Gibbs Phase Rule: F + P = C +2 Understanding Heterogeneous Equilibria: Determining F, establishing control... One-Component Diagrams: ?-? plots Two-Component Diagrams: ?-?? plots (eutectics, solid solutions, melting, ...) Chemical Vapor Transport Heterogeneous Solid-Gas Equilibria: growing high-quality crystals Kinetics: Transport rate Thermodynamics: Transport direction (hot-to-cold; cold-to-hot) Thermodynamics: Optimum median temperature 56