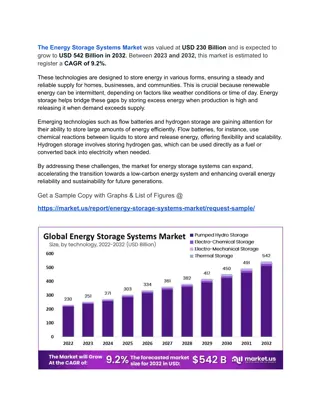
Chemistry Challenge: Chemical Storage & Pipets Usage
In chemistry, it's crucial to handle chemicals safely. Make sure to return all unused chemicals back to their original containers to prevent accidents. Pipets play a vital role in measuring and dispensing small liquid amounts accurately. Stay informed and practice proper lab procedures for a successful chemistry experiment.
Uploaded on Feb 22, 2025 | 0 Views
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Chemistry Aug 24, 2016 P3 Challenge Do Now True or False 1) Return all unused chemicals to their original containers. 2) Pipets are used to measure and dispense small amounts of liquids. You should draw the liquid into the pipet using your mouth. 3) All chemicals in the lab are to be considered dangerous. 4) All unauthorized experiments are prohibited.
Objectives and Agenda Objectives To Understand the Scientific Method To Use Significant Digits Agenda Chemistry Defined and the Nature of Science Significant Digits Experimental Design Scientific Notation Data Analysis Significant Digits in Calculations Laws and Theories
Todays Assignment What s Due? (Pending assignments to complete.) Significant Figure Practice Worksheet What s Next? (How to prepare for the next day) Read p46 - 59
Chemistry Defined Chemistry is the scientific study of matter, its properties, and its changes. Scientific Study . using the scientific method Of Matter . anything that has mass and takes up space Mass and weight Its Properties what can we measure Its Changes how does matter change over time
Nature of Science Observation Quantitative observations (measurements) Qualitative observations (descriptions) Communication Without sharing information, observations are useless to advance human knowledge Replication Over time, place and observer Falsifiable, Testable Serendipity and Chance discoveries Penicillium, Teflon, X-rays
Experimental Design Determine what factors or variables you are interested in studying. Identify Independent and dependent variables. (IV and DV) Your experiment may have more than one of each. An IV is one that you can manage and set levels or cases. A DV is one that you observe.
Experimental Design A control is one cases where the IV is absent, yet all other features of the experiment remain the same, and the DV is observed. Controls necessary to show measurable effects, to rule out other explanations, to allow for conclusions to be drawn. A hypothesis is a guess about the relationship between your variables of interest. Science is not a linear process. See also Figure 8 on pg 46.
Data Analysis After you perform the experimental trials as outlined by your design, you will have a data set to analyze with several observations of the same event. Average result Compare to a known or expected result using a percent error. % ????? =??????? ???????? ??? ???????? Range = Max observation Min observation. May use a graph to show relationships between variables.
Accuracy and Precision Accuracy low % error how close is the Average to the Expected Precision small range how close are the data to each other
Evaluating Results Two Sources of error Systematic errors Random errors Systematic (should be minimized with careful attention to lab techniques) Instrumental (Ex: Every piece of measuring glassware has an uncertainty associated with it. Marked on the glassware.) Procedural (Ex: During a chemical processing, every transfer from one vessel to another creates some additional error) Tends to influence resulting data to be skewed in one direction
Evaluating Results Random Can never remove uncertainty because of the fundamentally random nature of the motion of atoms. Influence of the observer (uncertainty principle) Tends to increase uncertainty of a measurement in all directions. Error vs a mistake
Laws and Theories A scientific law is a statement or mathematical equation that reliably describes a behavior of the natural world. Ex: Law of Conservation of Mass Antoine Lavoisier Summarizes macroscopic and atomic phenomena (What happens) A scientific theory is a well-tested explanation of observations. Ex: John Dalton s Atomic Theory Explains macroscopic and atomic phenomena (Why does it happen) Conflicting Info for a theory Revise or replace
How to measure Uncertainty depends on the measurement device used and on how it is used to measure. Identify the smallest gradation marked on the measurement device. Measure to this level of precision (certain measurement) Add one more digit as an estimation of the true measurement s location between the smallest gradation marks. (source of uncertainty) Try to decide on one of the five statements below. If you can t decide between two statements, use the odd digit between. 0 right on the line, 2 just above the line, 4 almost halfway, 6 a little more than halfway, 8 nearly to the next line.
Exact and Inexact Numbers Counting items gives an exact number (e.g. 12 apples = exactly 12 apples) Definitions within a measurement system give exact numbers (e.g. exactly 12 inches = exactly 1 foot, by definition) Measurements give inexact numbers (e.g. 5.7 m) In science, we don t use fractions. Conversion factors between systems of measurements give inexact numbers. (e.g. 1 lb = approximately 0.4536 kg to 4 sigfigs) Agreed uncertainty is 1 for the digit in the last decimal place reported or measured 5.7 m means 5.7 0.1 m 940 m means 940 10 m 2.54 cm means 2.54 0.01 cm
Determining Significant Figures The digits of a measured number indicate the level of precision for the measurement. All non-zero numbers are significant. Leading zeros are never significant. Confined zeros are always significant. Trailing zeros are significant if and only if a decimal point is present. Ambiguous numbers like 300 mL can be made clear by using a decimal point, a line over the last significant digit, or better, use scientific notation.
Practice identifying sigfigs 40.7 L 0.095 987 m 250. cm 87 009 km 85.00 g 9.000 000 000 mm 0.0009 kg 2000 cm
Scientific Notation A clear way to report numbers that are either very large or very small, or numbers with an ambiguous number of significant figures Two parts: Mantissa x 10exponent Mantissa, or decimal part, is a number with one non-zero digit to the left of the decimal point that contains all significant digits, and only significant digits Exponent indicates the decimal place location for the digits. Exponent = number of places the decimal point must move to be placed to the right of the first significant figure Large numbers have positive exponents and small numbers have negative exponents. 2000 cm 2.00 x 103cm makes the significance clear. Use Sci Not if you need clarity or you need to use more than 3 zeros.
Multiplication/Division with Significant Figures Perform the calculation indicated. Round your answer to the same number of significant figures as the number with the least number of significant figures that was used in the calculation. DO NOT report all digits shown in your calculator Round down for 4 or below. Round up for 5 and above. If a data set has a lot of rounding from 5, you may want to use engineering rounding: Round to an even digit result. Ex: 1.92 m x 11.53 m = 22.1376 m2 How should this be rounded?
Addition/Subtraction with Significant Figures Perform the calculation indicated. Round your answer to the same number of decimal places as the number with the least precise decimal place that was used in the calculation. DO NOT report all digits shown in your calculator The number of significant figures may increase or decrease. Hint: Remove any scientific notation, then add/subtract numbers vertically. The place with a significant digit for all numbers is the place to round your answer. Ex: 0.92 m + 1.503 m = 2.423 m How should this be rounded?
Multistep problems with mixed functions In general, keep extra digits for intermediate results, while at the same time noting which place is significant by underlining that digit. Ex: 3.252 (0.125 + 1.30) = 3.252 (1.425) = 4.6341 Rounds to 4.63. Why? A) 1.425 is only significant to two decimal places because it was the result of an addition between 3 d.p and 2 d.p. That gives it 3 sigfigs. B) The last multiplication is between a number with 4 sigfigs and 3 sigfigs, so the answer is rounded to 3 sigfigs. Ex: 3.252 x 0.125 + 1.30 = 0.4065 + 1.30 = 1.7065 How should this be rounded? Often it is easiest to just not clear your calculator for intermediate results. Rule of thumb: Follow significance throughout, but don t round until the end.
Exit Slip - Homework 1) Sig fig rule for Mult/Div. Round to . 2) Sig fig rule for Add/Sub. Round to What s Due? (Pending assignments to complete.) Significant Figure Practice Worksheet What s Next? (How to prepare for the next day) Read p46 - 59