Chemistry Lecture Announcements and Stoichiometry Concepts
In this lecture, the announcements cover important class updates and quizzes, while delving into the fundamental concept of stoichiometry in chemistry. Stoichiometry involves understanding the ratios of reactants and products in chemical reactions. The text explores error and uncertainty in measurements, significant figures, and the methods for delivering known amounts of reagents. Practical examples illustrate how to apply stoichiometry principles to solve problems effectively. The content also discusses different measures of uncertainty in scientific measurements, emphasizing the importance of precision and accurate data interpretation.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Announcements I Adding If you received an add slip, turn it into the office soon (so I know status of lab sections) Turn in today Corrected diagnostic quiz Quiz 1 Today (after announcements) Lab Procedures Quiz Thursday and next Monday (sect. 5, 6, and 7 are now ahead)
Announcements II Websites Text homework solutions posted (class website) SacCT site is set up (only grading columns so far; but I will post the diagnostic quiz and quiz 1 solutions soon) Today s Lecture Stoichiometry (Chapter 1) Error and Uncertainty (Chapter 3) Definitions Significant figures Accuracy and precision in measurements
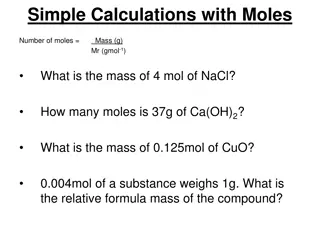
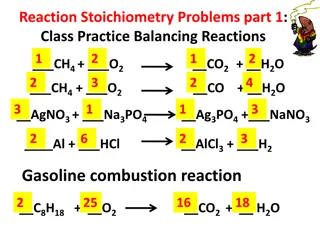
Stoichiometry Stoichiometry refers to ratios between moles of reactants and products in chemical reactions The ratio of moles of reactants and products is equal to the ratio of their stoichiometric coefficients Example: aA + bB cC + dD Moles A/moles B = a/b
Stoichiometry Example problem: How many moles of H2O2are needed to completely react with 25 mL of 0.80 M MnO4-? Reaction: 5H2O2(aq) + 2MnO4-+6H+ 2Mn2++ 5O2(g) + 8H2O(l)
Stoichiometry Remember: there are two (common) ways to deliver a known amount (moles) of a reagent: Mass (using formula weight) Volume (if molarity is known)
Chapter 3 Error and Uncertainty Error is the difference between measured value and true value or error = measured value true value Uncertainty Less precise definition The range of possible values that, within some probability, includes the true value
Measures of Uncertainty Explicit Uncertainty: Measurement of CO2in the air: 399 + 3 ppmv The + 3 ppm comes from statistics associated with making multiple measurements (Covered in Chapter 4) Implicit Uncertainty: Use of significant figures (399 has a different meaning than 400 and 399.32)
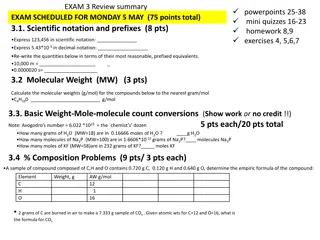
Significant Figures (review of general chem.) Two important quantities to know: Number of significant figures Place of last significant figure Example: 13.06 4 significant figures and last place is hundredths Learn significant figures rules regarding zeros
Significant Figures - Review Some Examples (give # of digits and place of last significant digit) 21.0 0.030 320 10.010
Significant Figures in Mathematical Operations Addition and Subtraction: Place of last significant digit is important (NOT number of significant figures) Place of sum or difference is given by least well known place in numbers being added or subtracted Example: 12.03 + 3 = 15.03 = 15 Hundredths place ones place Least well known
Significant Figures in Mathematical Operations Multiplication and Division Number of sig figs is important Number of sig figs in Product/quotient is given by the smallest # of sig figs in numbers being multiplied or divided Example: 3.2 x 163.02 = 521.664 = 520 = 5.2 x 102 2 places 5 places
Significant Figures in Mathematical Operations Multi-step Calculations Follow rules for each step Keep track of # of and place of last significant digits, but retain more sig figs than needed until final step Example: (27.31 22.4)2.51 = ? Step 1 (subtraction): (4.91)2.51 Note: 4.91 only has 2 sig figs, more digits listed (and used in next step) Step 2 multiplication = 12.3241 = 12
Significant Figures More Rules Separate rules for logarithms and powers (Covering, know for homework, but not tests) logarithms: # sig figs in result to the right of decimal point = # sig figs in operand example: log(107) = 2.02938 = 2.029 results need 3 sig figs past decimal point 107 = operand 3 sig fig Powers: # sig figs in results = # sig figs in operand to the right of decimal point example: 10-11.6 = 2.51 x 10-12 = 3 x 10-12 1 sig fig past decimal point
Types of Errors True Volume Systematic Errors Always off in one direction Examples: using a stretched plastic ruler to make length measurements (true length is always greater than measured length); reading buret without moving eye to correct height Random Errors Equally likely in any direction Present in any (continuously varying type) measurement Examples: 1) fluctuation in readings of a balance with window open, 2) errors in interpolating (reading between markings) buret readings eye Meas. Volume
Accuracy and Precision Accuracy is a measure of how close a measured value is to a true value Precision is a measure of the variability of measured values Precise, but not accurate Poor precision (Accuracy Precise and Accurate also not great)
Accuracy and Precision Accuracy is affected by systematic and random errors Precision is affected mainly by random errors Precision is easier to measure
Accuracy and Precision Both imprecise and inaccurate measurements can be improved Accounting for errors improves inaccurate measurements (if shot is above and right aim low + left) Averaging improves imprecise measurements rough ave of imprecise shots aim here