Chemistry Lesson: Molar Mass of Fe(NO3)3 & Empirical Formulas
In this chemistry lesson, you will explore the determination of the molar mass of Fe(NO3)3, learn about mass percentage, and delve into empirical formulas using examples and practice exercises. Understand how to calculate mass percentages, determine empirical formulas, and work with fractions and decimals in chemical formulas. Gain insights into mass percent composition, empirical formulas, and converting mass to moles for chemical analysis.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
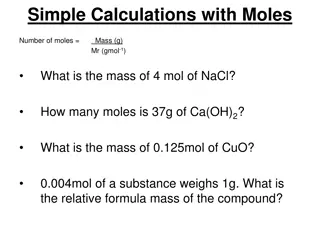
Chemistry Week of April 13 Lesson #2 P3 Challenge Determine the molar mass of Fe(NO3)3 Today s Objective Mass Percentage and Empirical Formulas Agenda Mass percent (review) Mass percent from formulas Assignment: Empirical formula from mass percent Empirical Formula Worksheet Molecular formula
Mass Percent Composition (Review) Given the number of grams of each element in a sample of a compound, you can calculate the mass percent composition. massofelement massofcompound = 100 masspercent Ex: Experiment shows a 43.8 g sample of sodium chloride contains 17.2 g of sodium. What is the mass percentage of each element in sodium chloride?
Determine mass percentage from formula Because a chemical formula represent relationships of moles of elements, you can determine the mass percent of any substance using molar masses. No experiment is needed!
Sample Percentage from Formula Determine the percent composition of each element in Al2O3 26.982 1 1 mol 26.982 g g Al Al mol Al Al = 53.964 53.964 g g Al Al 2 mol mol Al Al 101.961g g x 100 = 52.93 52.93 % % Al Al 101.961 15.999 ? mol 15.999 g g O O mol O O = 47.997 47.997 g g O O 101.961 3 mol mol O O 101.961 g g x 100 = ??.??% % O O
Practice Percentage from Formula Ex: Find the mass percentage of each element in NaCl. Verify that you get the same results we got from an experimental analysis. (39.3% Na, 60.7%Cl) Na ??.??? g g Na 1 1 mol Na = 22.990 22.990 g g Na 58.44 Na 58.44 g g x 100 ? mol mol Na = 39.34 39.34 % % Na Na mol Na Na = 35.45 35.45 g g Cl Cl 58.44 35.45 ? mol 35.45 g g Cl Cl mol Cl Cl 58.44 g g x 100 = 60.66 60.66 % % Cl Cl ? mol mol Cl Cl
Determining Empirical Formulas If you know the mass percentage of a compound, you can determine its chemical formula, most of the time. (Go backwards) Because this chemical formula is found from data, it is an empirical formula. Assume you have 100 g of a substance and write the percent composition in terms of grams of each element Convert these masses to moles. Divide by the smallest result. You should get integers. (If you get a decimal, multiply all by a factor to get integers.) Write your empirical formula. Often you can name the result. 1. 2. 3. 4. 5.
Fractions and Decimals When you divide by the smallest number of moles, you won t always get integers. If you get a decimal quantity, it reveals a different ratio based on halves, thirds, quarters etc Use this table to decide what to multiply by to get integers. Fraction form 1/2 1/3, or 2/3 1/4, or 1/5, 2/5, 3/5, 4/5 Decimal form 0.5 0.333, 0.666 0.25, 0.75 0.2, 0.4, 0.6, 0.8 Multiply by 2 3 4 5
Sample Empirical Formula Problem A compound is found to contain 13.42% Al, 38.81% Cr, and 47.77% O. Determine the empirical formula for this compound. 1 1 mol mol Al Al 26.982 26.982 g g Al Al = 0.4974 0.4974 g g Al Al 0.4974 ??.?? g g Al Al 0.4974 g g = 1 1 Al Al ? = 2 2 Al Al Al2Cr3O12 1 1 mol mol Cr 51.996 51.996 g g Cr Cr ??.?? g g Cr Cr = 0.7464 0.7464 g g Cr 0.4974 Cr 0.4974 g g Cr = 1.5 1.5 Cr Cr ? = 3 3 Cr Cr Al2(CrO4)3 1 1 mol mol O O 15.999 15.999 g g O O ??.?? g g O O = 2.986 2.986 g g O O Aluminum Chromate = 6 6 O O ? = ?2 2 O O 0.4974 0.4974 g g
Determine Empirical Formula You can also use experimental masses. Ex: A particular sample contains 9.56 g Al, 17.04 g S, and 25.30 g O. What is the empirical formula of this substance? 1 1 mol mol Al Al 26.982 26.982 g g Al Al = 0.3543 0.3543 g g Al Al 0.3543 ?.?? g g Al Al 0.3543 g g = 1 1 Al Al ? = 2 2 Al Al Al2S3O9 1 1 mol mol S S 32.06 32.06 g g S S ??.?? g g S S = 0.5315 0.5315 g g S S 0.3543 0.3543 g g = 1.5 1.5 S S ? = 3 3 S S Al2(SO3)3 1 1 mol mol O O 15.999 15.999 g g O O ??.?? g g O O = 1.581 1.581 g g O O 0.3543 0.3543 g g Aluminum Sulfite = 4.5 4.5 O O ? = 9 9 O O
Empirical Formula Most of the time, an empirical formula is the same as a molecular chemical formula, but not always. Consider a compound A that is 40.0% carbon, 6.7 % hydrogen and 53.3% oxygen. Determine the empirical formula of this compound. CH2O Now what if I told you the molar mass of this compound is 180 g/mol?
From Empirical Formula to Molecular Formula In order to determine the molecular formula, you also need to know the molar mass of the compound. Divide the molar mass by the molar mass of the empirical formula. Multiply your empirical formula by this factor to determine the molecular formula. For our last problem, 180 g/mol / 30g/mol = 6. C6H12O6 1. 2.
Exit Slip - Homework Exit Slip: A compound is found to contain 55.27% Sn, 14.93% S, and 29.80% O. Determine the empirical formula for this compound. Then name the substance. SnSO4 tin (II) sulfate What s Due? Empirical Formula worksheet