Chromatography Techniques and Principles Explained
Explore the fascinating world of chromatography - a method used to separate and identify components of mixtures. Learn about different types like paper, thin-layer, and gas-liquid chromatography, as well as the essential components needed for chromatographic separations. Discover how thin-layer chromatography (TLC) works to compare samples, and delve into the intricacies of gas-liquid chromatography (GLC). Understand the practical aspects, retention time factors, and interpretation of chromatograms. Unravel the mysteries of chromatography in this comprehensive guide.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Chemical Ideas 7.6 Chromatography
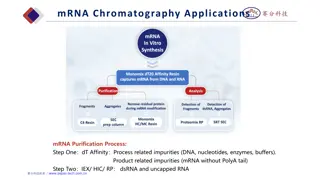
The general principle. Use to separate and identify components of mixtures. Several different types - paper, thin layer, gas-liquid. All use the principle of partition - affinity between two phases, to separate mixtures of substances. Stationary phase & mobile phase. Compounds with greatest affinity for mobile phase travel further.
All chromatography needs: support material stationary phase solvent (or carrier gas) mobile phase. Chromatography Type Paper Stationary phase Mobile phase Paper Organic or aqueous solvent. Organic or aqueous solvent. Thin layer Silica gel supported on plastic film High boiling point liquid on inert solid support. G.L.C. Inert gas e.g. nitrogen.
Thin Layer Chromatography - t.l.c. Series of spots forms Compare samples in mixture with known substances. Measure Rfvalues. Coloured compounds & colourless compounds.
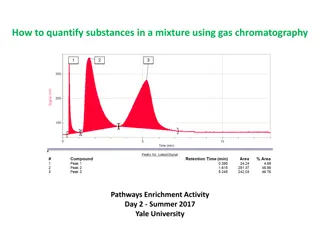
Gas - Liquid Chromatography G.l.c. Sample introduced by syringe. Column separates components. (Heated in oven) Detector monitors compounds emerging from outlet. Recorder plots signals as a chromatogram.
What happens in practice. Compounds that have high affinity for mobile phaseemerge first, (most volatile). Chromatogram charts recorder response against time. Each component - separate peak. Retention time characteristic of the compound under given conditions.
Factors affecting retention time: length of column packing material type of carrier gas flow rate of carrier gas temperature of column.
Interpreting the trace Calibration known compounds are added to the column and conditions kept constant. Amount of substance area under peak / peak height. Relative proportions can be determined.
Uses of G.l.c. Very sensitive - small quantities of substances detected, explosives, drugs etc. Separation of pure substances for collection. Can be connected to mass spectrometer for direct identification of substances.
This powerpoint was kindly donated to www.worldofteaching.com http://www.worldofteaching.com is home to over a thousand powerpoints submitted by teachers. This is a completely free site and requires no registration. Please visit and I hope it will help in your teaching.