Coagulation and Flocculation in Water Treatment
Learn about the process of coagulation and flocculation in water treatment, where suspended solids of varying sizes are removed to improve water quality. Discover the importance of colloidal particles, the role of Van der Waals forces, and the use of coagulants to achieve destabilization. Explore traditional coagulants like metal salts and modern approaches using polymers for charge neutralization.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
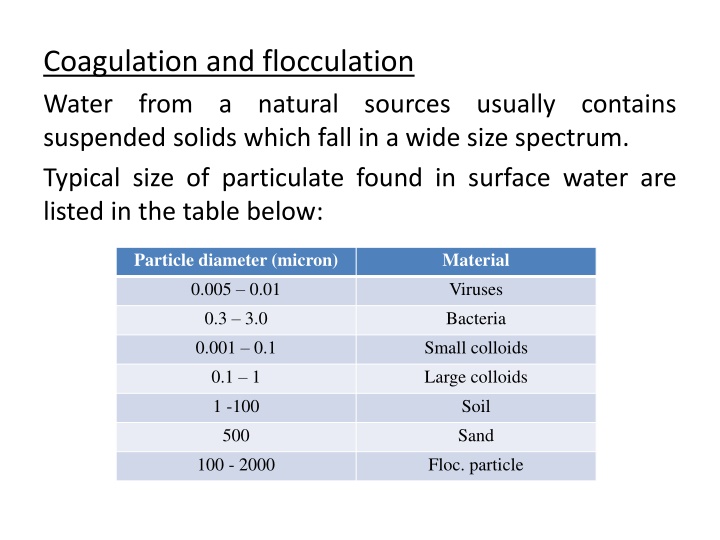
Coagulation and flocculation Water from a suspended solids which fall in a wide size spectrum. Typical size of particulate found in surface water are listed in the table below: natural sources usually contains Particle diameter (micron) 0.005 0.01 0.3 3.0 0.001 0.1 0.1 1 1 -100 500 100 - 2000 Material Viruses Bacteria Small colloids Large colloids Soil Sand Floc. particle
Particles greater than 1 micrometer will usually settle in quiescent water. Smaller particle will not settle readily and they are known as colloids. Characteristics of colloids Colloidal particles size is generally less than 1 micron such as a clay, silts (inorganics), bacteria, viruses, plankton (organics). Generally colloids in natural water have negative charge. Because this colloids have the same charge, they will repel each others.
Van Der walls forces is a force of attraction which exists between any two masses depending on the mass of the two bodies and the distance between them. Repulsion due to electrical charge will normally repel the colloids before they can move close enough for Van Der walls force to become significant. If the magnitude of the electrical force could be reduced, the particle would move close enough for Van Der walls force to predominant.
Coagulation Coagulation is a chemical treatment process used to destabilize colloidal particles. Coagulant is the chemical that is added to the water to achieve coagulation process. Traditionally, metal salts as aluminum sulfate (alum), ferric sulfate, ferric chloride and ferrous sulfate have been utilized as coagulants.
Recently polymers (long molecular chain organic compounds) have been coagulation process. The most important mechanism to achieve coagulation is charge neutralization , which means that the positive aluminum ions are adsorbed on the negatively charged colloids. Therefore the destabilized colloidal particles can adhere to each other to form colloidal colloidal complex. However, an excess addition of counter ionic materials may result in restabilization by a charge reversal. The net charge on the particle may be reversed by the adsorption of an excess of counter ions. used to enhance the
The excess metal salts (overdose of alum) hydrolyzed into the form of metal hydroxide, which are extremely insoluble in water. As the hydroxide precipitate, they sweep through the water containing colloidal particles. The colloidal particles that become enmeshed in the floc will thus be removed from the water. Rapid mixer should provide sufficient agitation to disperse the chemical thoroughly in the water. Rapid mixing unit utilizes mechanical mixer and they are usually square and have a depth to width ratio of approximately 2.
A Channel with fully turbulent flow of sufficient length to yield the desired detention time follower a hydraulic jump has been used successfully.
Flocculation The term flocculation is used to describe the process where the size of particles increases as a results of particle collision due to gentle mixing. The purpose of flocculation is to produce particles that can be removed by sedimentation, this could be achieved by providing particles contact while not creating sufficient turbulence to break up the flocs already formed.
Flocculant polymers) added to ensure the flocculation process. : is a chemical (typically organic The flocculant being a polymer has the ability to bridge particles by adsorption of the polymer onto the colloids the resulting structure grows as a single particle several times larger than its constituent. Bridging is a mechanism in which the polymer acts as a bridge between particles to produce a bigger flocs and excess dosage of polymer may cause restabilization of particles due to surface saturation.
The design objective in hydraulic flocculation is to achieve gentle uniform mixing that will not shear the flocs. The mixers typically used in flocculation basin are horizontal shaft Paddle-wheel flocculators. When paddle flocculators are used, the compartments are typically 6 - 30 m long and 3 - 5 m wide. For vertical flocculators dimensions of compartment are 6 x 6 meter and depth are 3 - 5 meter.
Flocculation basins are normally designed with multiple mixing compartment which called tapered flocculation. The first compartment causes a rapid transformation of the particles into higher density flocs, the subsequent compartment causes the build-up of large size flocs for better settling.
This design helps to: 1. produce uniform and tough flocs that will settle readily. 2. This design helps to minimize short-circuiting effectively 3. No need to extreme large paddle, three reasonable size paddle are enough.
Agitation requirement In order to quickly and completely homogenize the coagulant, the rapid mixer should be designed to provide a short period of violent agitation. Detention time in Rapid mixing tank is 10 seconds to 5 minutes. Regarding employed is much less than that used for Rapid mixing. Detention time in flocculation basin is much higher than that in rapid mixer, detention time for 20 - 60 min are common in flocculation tank. flocculation, the degree of agitation
Traditionally, in water treatment, the degree of agitation in a mixing unit is measured by velocity gradient. For mixing equipment, the value of the velocity gradient is given by: ? = (?/??) G= velocity gradient 1/s P:power imparted to the water N.m/s V:volume of the basin m3 ?: absolute viscosity of the fluid (N.S/m2)
In the case of paddle wheel mixer, the water power is given by: ?? ? ? ??3 2 ? = P: power imparted to water. CD: coefficient length/width ratio (L/W) of the paddle blade usually 1.8 for flat blades. A: area of paddles (m2). VP: velocity of the paddle relative to the water (m /sec). of drag, which varies with the
The approximately 75% of the absolute peripheral velocity of the paddle. The peripheral speed of a paddle wheel mixer should be limited to 0.4 m/s for alum. Less than 1m / s paddle velocity relative to the water is















