Colorful Flame Tests
Explore the fascinating world of flame tests in chemistry, where different metal atoms produce distinct colors when heated. Discover how flame tests are used to identify elements in compounds and delve into the atomic structure behind these colorful phenomena.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Lesson 17: Technicolor Atoms Flame Tests
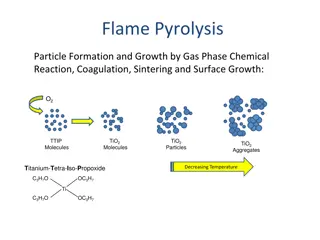
Discussion Notes The metal element in each chemical formula appears to be responsible for the flame colors. Only certain elements produce colorful flames. Flame test: A test used in the laboratory to look for the presence of certain metal atoms. A sample of a compound is heated in a flame, and the resulting color is noted.
Discussion Notes (cont.) Elements and compounds are collections of atoms. The only way to change one atom into another is to change the nucleus through a nuclear reaction.
Discussion Notes (cont.) Sodium Atom, Na
Discussion Notes (cont.) The illustration indicates that the flame colors are associated with movements of the electrons within the sodium atom. Bohr s model of the atom came directly from evidence similar to that produced in class today.
Wrap Up What evidence is there that certain atoms are present in a compound? Many metal atoms produce a characteristic colored flame when compounds containing those atoms are heated in a flame. Flame tests are evidence that elements and compounds are collections of atoms.
Check-In Given: Each compound consists of a metal and non-metal. Copper chloride (CuCl2) burns blue-green Sodium chloride (NaCl) burns bright orange Strontium chloride (SrCl2) burns red Potassium chloride (KCl) burns pink lilac Find: 1. What part of each compound above is responsible for producing the change in flame color? Predict what color a solution containing a mixture of CuCl2and SrCl2will burn in a flame test. Support your explanation with scientific thinking! 2.
Flame Test Analysis Qs OK to skip analysis questions 1-4 if data table 1 is complete and correct Complete Qs 5-7, make sure your name is on the packet, and turn it in now.