Common Names of Simple Aldehydes and Ketones
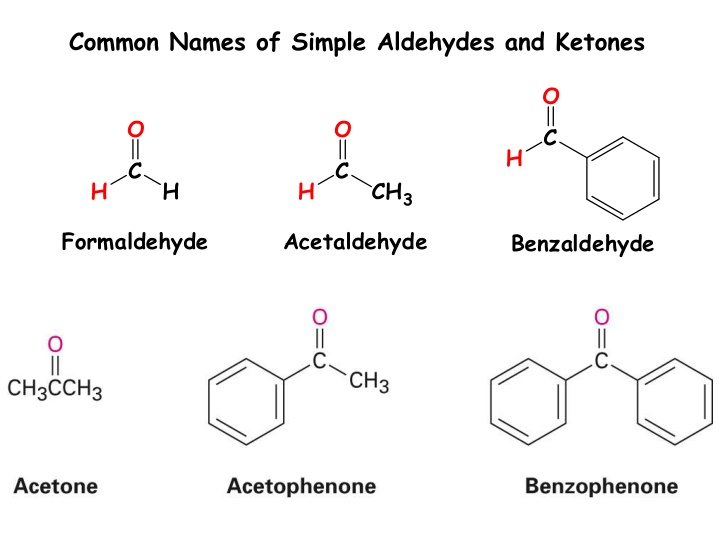
Common names of simple aldehydes like formaldehyde, acetaldehyde, and benzaldehyde, along with the preparation methods for aldehydes and ketones through oxidation of alcohols and other chemical reactions are discussed in this content. Methods such as oxidation using PCC and Dess-Martin Periodinane, as well as the transformation of 1 alcohols to aldehydes and 2 alcohols to ketones, are elaborated. Additionally, the preparation of ketones from acid chlorides and aldehydes from esters reduction is highlighted.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
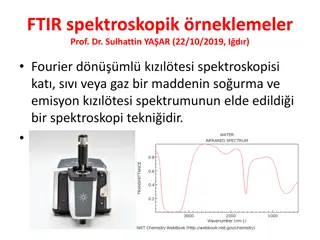
Common Names of Simple Aldehydes and Ketones O O O C H C C H H H CH3 Formaldehyde Acetaldehyde Benzaldehyde
Preparation of Aldehydes and Ketones : Review 1. Oxidation of 1 alcohols to aldehydes: a. PCC b. Dess-Martin Periodinane
Preparation of Aldehydes and Ketones : Review 1. Oxidation of 1 alcohols to aldehydes: a. PCC b. Dess-Martin Periodinane
Preparation of Aldehydes and Ketones : Review 2. Oxidation of 2 alcohols to ketones: a. CrO3 , H2SO4 b. K2Cr2O7, H2SO4 c. PCC d. Dess - Martin
Preparation of Aldehydes and Ketones : Review 2. Oxidation of 2 alcohols to ketones: a. CrO3 , H2SO4 b. K2Cr2O7, H2SO4 c. PCC d. Dess - Martin
Preparation of Aldehydes and Ketones : Review 3. Aryl aldehydes and ketones: Friedel Crafts Acylation
Preparation of Aldehydes and Ketones : Review 3. Aryl aldehydes and ketones: Friedel Crafts Acylation
Preparation of Aldehydes and Ketones: 1. Ketones From Acid Chlorides via Acyl Transfer Using R2CuLi (Section 16.4)
Preparation of Aldehydes and Ketones: 1. Ketones From Acid Chlorides via Acyl Transfer Using R2CuLi (Section 16.4) 2. Aldehydes From Esters Reduction with DIBAH (Section 16.6)
Oxidation of Aldehydes : Review a. CrO3, H2SO4 b. K2Cr2O7, H2SO4
Oxidation of Aldehydes : Review a. CrO3, H2SO4 b. K2Cr2O7, H2SO4
Nucleophilic Additions to Aldehydes and Ketones I. Reactivity 1. Aldehydes vs. ketones
Nucleophilic Additions to Aldehydes and Ketones I. Reactivity 1. Aldehydes vs. ketones 2. Aromatic vs. aliphatic
Nucleophilic Additions to Aldehydes and Ketones II. Types of Nucleophiles 1. Anionic 2. Neutral
Nucleophilic Additions to Aldehydes and Ketones II. Types of Nucleophiles 1. Anionic
Nucleophilic Additions to Aldehydes and Ketones II. Types of Nucleophiles 1. Anionic 2. Neutral i. Water a. hydrates Acid catalyzed Base catalyzed
Nucleophilic Additions to Aldehydes and Ketones II. Types of Nucleophiles 1. Anionic 2. Neutral i. Water ii. Alcohols a. acetals
Nucleophilic Additions to Aldehydes and Ketones II. Types of Nucleophiles 1. Anionic 2. Neutral i. Water ii. Alcohols iii. 1 amines and hydrazines
Nucleophilic Additions to Aldehydes and Ketones II. Types of Nucleophiles 1. Anionic 2. Neutral i. Water ii. Alcohols iii. 1 amines and hydrazines a. imines
Nucleophilic Additions to Aldehydes and Ketones II. Types of Nucleophiles 1. Anionic 2. Neutral i. Water ii. Alcohols iii. 1 amines and hydrazines a. imines b. hydrazones c. Wolff-Kirshner Reduction
Nucleophilic Additions to Aldehydes and Ketones II. Types of Nucleophiles 1. Anionic 2. Neutral i. Water ii. Alcohols iii. 1 amines and hydrazines iv. 2 amines a. enamines
Professor Gilbert Stork Stork was born in Brussels, Belgium, and received his secondary education in France. The B.S. (1942) and Ph.D. (1945) were obtained at the Universities of Florida and Wisconsin respectively. He was on the Harvard faculty (1946-53), then joined the Columbia faculty where he was instrumental in building its strong organic group and is currently Eugene Higgins Professor Emeritus. Stork's many awards include the ACS Award in Pure Chemistry (1957), the ACS Award for Creative Work in Synthetic Organic Chemistry (1967), the Arthur C. Cope Award (1980), the Willard Gibbs Medal (1982), the National Medal of Science (1982), the Tetrahedron Prize (1985), the Roger Adams Award (1991) and the Wolf Prize (1996).In 2001 he published the first completely stereoselective total synthesis of quinine. http://chem.tufts.edu/AMichael-Bio.html
Reactions of Ylides with Aldehydes I. The Wittig Reaction 1. The Metathesis reaction George Wittig 1979 Nobel Prize
Reactions of Ylides with Aldehydes I. The Wittig Reaction 1. The Metathesis reaction 2. Generation and nature of ylides George Wittig 1979 Nobel Prize
Reactions of Ylides with Aldehydes I. The Wittig Reaction 1. The Metathesis reaction 2. Generation and nature of ylides 3. Stereochemistry a. effect of the ylide George Wittig 1979 Nobel Prize
Reactions of Ylides with Aldehydes I. The Wittig Reaction 1. The Metathesis reaction 2. Generation and nature of ylides 3. Stereochemistry a. effect of the ylide 4. The Mechanism a. oxaphosphatane formation b. elimination George Wittig 1979 Nobel Prize
Reactivity of -unsaturated carbonly compounds 1. Electrophilic sites
Reactivity of -unsaturated carbonly compounds 1. Electrophilic sites
Reactivity of -unsaturated carbonly compounds 1. Electrophilic sites 2. Hard-soft acid-base theory
Professor Author Michael Born in Buffalo NY in 1855, he studied chemistry at Heidelberg under Robert Bunsen (1811-1899) and at Berlin under August Wilhelm Hofmann (1818-1892). He then studied under Adolphe Wurtz (1817-1884) in Paris and Dimitri Ivanovi Mendeleev (1834-1907) in St Petersburg, but never bothered to take a degree. He was made Professor of Chemistry at Tufts College near Boston. In 1889, he married one of his most brilliant students, (1857-1904), one of the few women organic chemists Helen Cecilia DeSilver Abbott in this period. After a failed attempt to run the chemistry department at Clark University in Worcester, Massachusetts, in 1891, he spent three years working with his wife in his private laboratory on the Isle of Wight before returning to Tufts. After he retired from Tufts in 1907, Michael set up another private laboratory at Newton Center near Boston. In 1912 he was appointed a Professor of Chemistry, without lecturing duties, at Harvard University. Arthur Michael died in 1942. http://chem.tufts.edu/AMichael-Bio.html
Reactivity of -unsaturated carbonly compounds 1. Electrophilic sites 2. Hard-soft acid-base theory 3. Examples